Embryogenesis: pattern formation from a single cell
- PMID: 22303250
- PMCID: PMC3243344
- DOI: 10.1199/tab.0126
Embryogenesis: pattern formation from a single cell
Abstract
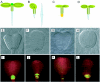
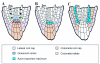
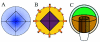
During embryogenesis a single cell gives rise to a functional multicellular organism. In higher plants, as in many other multicellular systems, essential architectural features, such as body axes and major tissue layers are established early in embryogenesis and serve as a positional framework for subsequent pattern elaboration. In Arabidopsis, the apicalbasal axis and the radial pattern of tissues wrapped around it are already recognizable in young embryos of only about a hundred cells in size. This early axial pattern seems to provide a coordinate system for the embryonic initiation of shoot and root. Findings from genetic studies in Arabidopsis are revealing molecular mechanisms underlying the initial establishment of the axial core pattern and its subsequent elaboration into functional shoots and roots. The genetic programs operating in the early embryo organize functional cell patterns rapidly and reproducibly from minimal cell numbers. Understanding their molecular details could therefore greatly expand our ability to generate plant body patterns de novo, with important implications for plant breeding and biotechnology.
Figures






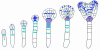







References
-
- Abe M., Takahashi T., Komeda Y. Cloning and characterization of an L1 layer-specific gene in Arabidopsis thaliana. Plant and Cell Physiology. 1999;407(1):571–580. - PubMed
-
- Abe M., Takahashi T., Komeda Y. Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J. 2001;267(1):487–494. - PubMed
-
- Abe M., Katsumata H., Komeda Y., T. Takahashi. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 2003;1307(1):635–643. - PubMed
-
- ]Aida M., Ishida T., Tasaka M. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development. 1999;1267(1):1563–1570. - PubMed
LinkOut - more resources
Full Text Sources
