Flower development
- PMID: 22303253
- PMCID: PMC3244948
- DOI: 10.1199/tab.0127
Flower development
Abstract
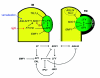
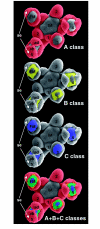
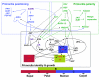
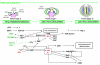
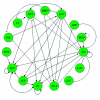
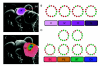
Flowers are the most complex structures of plants. Studies of Arabidopsis thaliana, which has typical eudicot flowers, have been fundamental in advancing the structural and molecular understanding of flower development. The main processes and stages of Arabidopsis flower development are summarized to provide a framework in which to interpret the detailed molecular genetic studies of genes assigned functions during flower development and is extended to recent genomics studies uncovering the key regulatory modules involved. Computational models have been used to study the concerted action and dynamics of the gene regulatory module that underlies patterning of the Arabidopsis inflorescence meristem and specification of the primordial cell types during early stages of flower development. This includes the gene combinations that specify sepal, petal, stamen and carpel identity, and genes that interact with them. As a dynamic gene regulatory network this module has been shown to converge to stable multigenic profiles that depend upon the overall network topology and are thus robust, which can explain the canalization of flower organ determination and the overall conservation of the basic flower plan among eudicots. Comparative and evolutionary approaches derived from Arabidopsis studies pave the way to studying the molecular basis of diverse floral morphologies.
Figures







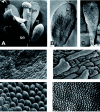






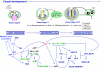



 ), including orders Ranunculales and Proteales and families Buxaceae, Sabiaceae and Trochodendraceae; Asparagales (
), including orders Ranunculales and Proteales and families Buxaceae, Sabiaceae and Trochodendraceae; Asparagales ( ) including Dioscorales, Liliales and Pandanales; (a) the Commelinid grade that, in addition to Poales and Commelinales, includes Dasipogonaeae, Arecales and Zingiberales; the Magnoliid complex (
) including Dioscorales, Liliales and Pandanales; (a) the Commelinid grade that, in addition to Poales and Commelinales, includes Dasipogonaeae, Arecales and Zingiberales; the Magnoliid complex ( ) including Canellales, Piperales, Laurales and Magnoliales. Images of rice spikelet, Nymphaea alba and the male Gnetum gnsmon reproductive structure were taken from Yale Virtual Centre for Cellular Expression Profiling of Rice
) including Canellales, Piperales, Laurales and Magnoliales. Images of rice spikelet, Nymphaea alba and the male Gnetum gnsmon reproductive structure were taken from Yale Virtual Centre for Cellular Expression Profiling of Rice References
-
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;3098(1):1052–1056. - PubMed
-
- Aida M., Tasaka M. Morphogenesis and patterning at the organ boundaries in the higher plant shoot apex. Plant Mol. Biol. 2006a;608(1):915–928. - PubMed
-
- Aida M., Tasaka M. Genetic control of shoot organ boundaries. Curr. Opin. Plant Biol. 2006b;98(1):72–77. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
