Photorespiration
- PMID: 22303256
- PMCID: PMC3244903
- DOI: 10.1199/tab.0130
Photorespiration
Abstract
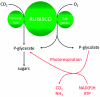
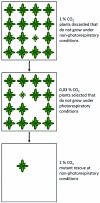
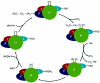
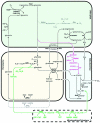
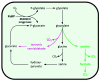
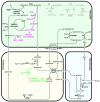
Photorespiration is initiated by the oxygenase activity of ribulose-1,5-bisphosphate-carboxylase/oxygenase (RUBISCO), the same enzyme that is also responsible for CO(2) fixation in almost all photosynthetic organisms. Phosphoglycolate formed by oxygen fixation is recycled to the Calvin cycle intermediate phosphoglycerate in the photorespiratory pathway. This reaction cascade consumes energy and reducing equivalents and part of the afore fixed carbon is again released as CO(2). Because of this, photorespiration was often viewed as a wasteful process. Here, we review the current knowledge on the components of the photorespiratory pathway that has been mainly achieved through genetic and biochemical studies in Arabidopsis. Based on this knowledge, the energy costs of photorespiration are calculated, but the numerous positive aspects that challenge the traditional view of photorespiration as a wasteful pathway are also discussed. An outline of possible alternative pathways beside the major pathway is provided. We summarize recent results about photorespiration in photosynthetic organisms expressing a carbon concentrating mechanism and the implications of these results for understanding Arabidopsis photorespiration. Finally, metabolic engineering approaches aiming to improve plant productivity by reducing photorespiratory losses are evaluated.
Figures


References
-
- Ainsworth E.A., Rogers A. The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant Cell Environ. 2007;308(1):258–270. - PubMed
-
- Anderson L.E. Chloroplast and cytoplasmic enzymes II. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta. 1971;2358(1):237–244. - PubMed
-
- Andersson I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008;598(1):1555–1568. - PubMed
-
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;558(1):373–399. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
