Proline metabolism and its implications for plant-environment interaction
- PMID: 22303265
- PMCID: PMC3244962
- DOI: 10.1199/tab.0140
Proline metabolism and its implications for plant-environment interaction
Abstract
Proline has long been known to accumulate in plants experiencing water limitation and this has driven studies of proline as a beneficial solute allowing plants to increase cellular osmolarity during water limitation. Proline metabolism also has roles in redox buffering and energy transfer and is involved in plant pathogen interaction and programmed cell death. Some of these unique roles of proline depend on the properties of proline itself, whereas others depend on the "proline cycle" of coordinated proline synthesis in the chloroplast and cytoplasm with proline catabolism in the mitochondria. The regulatory mechanisms controlling proline metabolism, intercellular and intracellular transport and connections of proline to other metabolic pathways are all important to the in vivo functions of proline metabolism. Connections of proline metabolism to the oxidative pentose phosphate pathway and glutamate-glutamine metabolism are of particular interest. The N-acetyl glutamate pathway can also produce ornithine and, potentially, proline but its role and activity are unclear. Use of model systems such as Arabidopsis thaliana to better understand both these long studied and newly emerging functions of proline can help in the design of next-generation experiments testing whether proline metabolism is a promising metabolic engineering target for improving stress resistance of economically important plants.
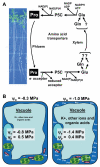
Figures
Similar articles
-
Proline Coordination with Fatty Acid Synthesis and Redox Metabolism of Chloroplast and Mitochondria.Plant Physiol. 2016 Oct;172(2):1074-1088. doi: 10.1104/pp.16.01097. Epub 2016 Aug 10. Plant Physiol. 2016. PMID: 27512016 Free PMC article.
-
A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical bio-synthesis and mechanism of action for human health and environmental applications.Asia Pac J Clin Nutr. 2004;13(1):1-24. Asia Pac J Clin Nutr. 2004. PMID: 15003910 Review.
-
Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens.Front Plant Sci. 2015 Jul 6;6:503. doi: 10.3389/fpls.2015.00503. eCollection 2015. Front Plant Sci. 2015. PMID: 26217357 Free PMC article. Review.
-
Ornithine-delta-aminotransferase and proline dehydrogenase genes play a role in non-host disease resistance by regulating pyrroline-5-carboxylate metabolism-induced hypersensitive response.Plant Cell Environ. 2012 Jul;35(7):1329-43. doi: 10.1111/j.1365-3040.2012.02492.x. Epub 2012 Mar 8. Plant Cell Environ. 2012. PMID: 22321246
-
Proline metabolism and cancer: emerging links to glutamine and collagen.Curr Opin Clin Nutr Metab Care. 2015 Jan;18(1):71-7. doi: 10.1097/MCO.0000000000000121. Curr Opin Clin Nutr Metab Care. 2015. PMID: 25474014 Free PMC article. Review.
Cited by
-
Light Control of Salt-Induced Proline Accumulation Is Mediated by ELONGATED HYPOCOTYL 5 in Arabidopsis.Front Plant Sci. 2019 Dec 10;10:1584. doi: 10.3389/fpls.2019.01584. eCollection 2019. Front Plant Sci. 2019. PMID: 31921239 Free PMC article.
-
Proline, Cysteine and Branched-Chain Amino Acids in Abiotic Stress Response of Land Plants and Microalgae.Plants (Basel). 2023 Sep 28;12(19):3410. doi: 10.3390/plants12193410. Plants (Basel). 2023. PMID: 37836150 Free PMC article. Review.
-
Abscisic acid mediated proline biosynthesis and antioxidant ability in roots of two different rice genotypes under hypoxic stress.BMC Plant Biol. 2020 May 8;20(1):198. doi: 10.1186/s12870-020-02414-3. BMC Plant Biol. 2020. PMID: 32384870 Free PMC article.
-
Arabidopsis AMINO ACID PERMEASE1 Contributes to Salt Stress-Induced Proline Uptake from Exogenous Sources.Front Plant Sci. 2017 Dec 22;8:2182. doi: 10.3389/fpls.2017.02182. eCollection 2017. Front Plant Sci. 2017. PMID: 29312416 Free PMC article.
-
Transcriptomic and Metabolomic Changes Triggered by Fusarium solani in Common Bean (Phaseolus vulgaris L.).Genes (Basel). 2020 Feb 7;11(2):177. doi: 10.3390/genes11020177. Genes (Basel). 2020. PMID: 32046085 Free PMC article.
References
-
- Abraham E., Rigo G., Szekely G., Nagy R., Koncz C., Szabados L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Molecular Biology. 2003;51:363–372. - PubMed
-
- Alcazar R., Altabella T., Marco F., Bortolotti C., Reymond M., Koncz C., Carrasco P., Tiburcio A.F. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–1249. - PubMed
-
- Armengaud P., Thiery L., Buhot N., Grenier-de March G., Savoure A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiologia Plantarum. 2004;120:442–450. - PubMed
LinkOut - more resources
Full Text Sources
