Plant ABC Transporters
- PMID: 22303277
- PMCID: PMC3268509
- DOI: 10.1199/tab.0153
Plant ABC Transporters
Abstract
ABC transporters constitute one of the largest protein families found in all living organisms. ABC transporters are driven by ATP hydrolysis and can act as exporters as well as importers. The plant genome encodes for more than 100 ABC transporters, largely exceeding that of other organisms. In Arabidopsis, only 22 out of 130 have been functionally analyzed. They are localized in most membranes of a plant cell such as the plasma membrane, the tonoplast, chloroplasts, mitochondria and peroxisomes and fulfill a multitude of functions. Originally identified as transporters involved in detoxification processes, they have later been shown to be required for organ growth, plant nutrition, plant development, response to abiotic stresses, pathogen resistance and the interaction of the plant with its environment. To fulfill these roles they exhibit different substrate specifies by e.g. depositing surface lipids, accumulating phytate in seeds, and transporting the phytohormones auxin and abscisic acid. The aim of this review is to give an insight into the functions of plant ABC transporters and to show their importance for plant development and survival.
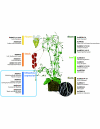
Figures





References
-
- Aarts M.G., Hodge R., Kalantidis K., Florack D., Wilson Z.A., Mulligan B.J., Stiekema W.J., Scott R., Pereira A. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J. 1997;12:615–623. - PubMed
-
- Akiyama K., Matsuzaki K., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. - PubMed
-
- Ali-Rachedi S., Bouinot D., Wagner M-H., Bonnet M., Sotta B., Grappin P., Julien M. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds; studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
