Maternal omega-3 supplementation increases fat mass in male and female rat offspring
- PMID: 22303344
- PMCID: PMC3268601
- DOI: 10.3389/fgene.2011.00048
Maternal omega-3 supplementation increases fat mass in male and female rat offspring
Abstract
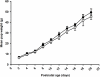
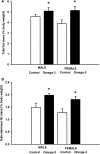
Adipogenesis and lipogenesis are highly sensitive to the nutritional environment in utero and in early postnatal life. Omega-3 long chain polyunsaturated fatty acids (LCPUFA) inhibit adipogenesis and lipogenesis in adult rats, however it is not known whether supplementing the maternal diet with omega-3 LCPUFA results in reduced fat deposition in the offspring. Female Albino Wistar rats were fed either a standard chow (Control, n = 10) or chow designed to provide ∼15 mg/kg/day of omega-3 LCPUFA, chiefly as docosahexaenoic acid (DHA), throughout pregnancy and lactation (Omega-3, n = 11) and all pups were weaned onto a commercial rat chow. Blood and tissues were collected from pups at 3 and 6 weeks of age and weights of visceral and subcutaneous fat depots recorded. The expression of adipogenic and lipogenic genes in the subcutaneous and visceral fat depots were determined using quantitative real time reverse transcription-PCR. Birth weight and postnatal growth were not different between groups. At 6 weeks of age, total percentage body fat was significantly increased in both male (5.09 ± 0.32% vs. 4.56 ± 0.2%, P < 0.04) and female (5.15 ± 0.37% vs. 3.89 ± 0.36%, P < 0.04) offspring of omega-3 dams compared to controls. The omega-3 LCPUFA content of erythrocyte phospholipids (as a% of total fatty acids) was higher in omega-3 offspring (6.7 ± 0.2% vs. 5.6 ± 0.2%, P < 0.001). There was no effect of maternal omega-3 LCPUFA supplementation on the expression of adipogenic or lipogenic genes in the offspring in either the visceral or subcutaneous fat depots. We have therefore established that an omega-3 rich environment during pregnancy and lactation in a rodent model increases fat accumulation in both male and female offspring, particularly in subcutaneous depots, but that this effect is not mediated via upregulation adipogenic/lipogenic gene transcription. These data suggest that maternal n-3 LCPUFA supplementation during pregnancy/lactation may not be an effective strategy for reducing fat deposition in the offspring.
Keywords: adipose tissue; maternal nutrition; omega-3.
Figures
References
-
- Ailhaud G., Massiera F., Weill P., Legrand P., Alessandri J. M., Guesnet P. (2006). Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 45, 203–236 10.1016/j.plipres.2006.01.003 - DOI - PubMed
-
- Armitage J. A., Khan I. Y., Taylor P. D., Nathanielsz P. W., Poston L. (2004). Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J. Physiol. (Lond.) 561, 355–377 10.1113/jphysiol.2004.072009 - DOI - PMC - PubMed
-
- Asserhoj M., Nehammer S., Matthiessen J., Michaelsen K. F., Lauritzen L. (2009). Maternal fish oil supplementation during lactation may adversely affect long-term blood pressure, energy intake, and physical activity of 7-year-old boys. J. Nutr. 139, 298–304 - PubMed
LinkOut - more resources
Full Text Sources

