Dystrophin Orchestrates the Epigenetic Profile of Muscle Cells Via miRNAs
- PMID: 22303359
- PMCID: PMC3268617
- DOI: 10.3389/fgene.2011.00064
Dystrophin Orchestrates the Epigenetic Profile of Muscle Cells Via miRNAs
Abstract
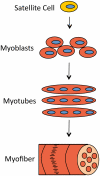
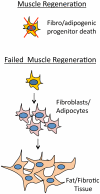
Mammalian musculature is a very robust and dynamic tissue that goes through many rounds of degeneration and regeneration in an individual's lifetime. There is a biological program that maintains muscle progenitor cells that, when activated, give rise to intermediate myoblast progeny that consequently differentiate into mature muscle cells. Recent works have provided a picture of the role that microRNAs (miRNAs) play in maintaining aspects of this program. Intriguingly, a subset of these miRNAs is de-regulated in muscular dystrophies (MDs), a group of fatal inherited neuromuscular disorders that are often associated with deficiencies in the Dystrophin (Dys) complex. Apparently, transcriptional expression of many of the muscle specific genes and miRNAs is dependent on chromatin state regulated by the Dys-Syn-nNOS pathway. This puts Dystrophin at the epicenter of a highly regulated program of muscle gene expression in which miRNAs help to coordinate networking between multiple phases of muscle maintenance, degeneration, and regeneration. Therefore, understanding the role of miRNAs in physiology of normal and diseased muscle tissue could be useful for future applications in improving the MD therapies and could open new clinical perspectives.
Keywords: Dys–Syn–nNOS; dystrophin; epigenetic regulation; miRNAs; muscular dystrophy.
Figures
Similar articles
-
Dg-Dys-Syn1 signaling in Drosophila regulates the microRNA profile.BMC Cell Biol. 2012 Oct 29;13:26. doi: 10.1186/1471-2121-13-26. BMC Cell Biol. 2012. PMID: 23107381 Free PMC article.
-
miR-708-5p and miR-34c-5p are involved in nNOS regulation in dystrophic context.Skelet Muscle. 2018 Apr 27;8(1):15. doi: 10.1186/s13395-018-0161-2. Skelet Muscle. 2018. PMID: 29703249 Free PMC article.
-
MiRNAs and Muscle Regeneration: Therapeutic Targets in Duchenne Muscular Dystrophy.Int J Mol Sci. 2021 Apr 19;22(8):4236. doi: 10.3390/ijms22084236. Int J Mol Sci. 2021. PMID: 33921834 Free PMC article. Review.
-
Genetic suppression of phenotypes arising from mutations in dystrophin-related genes in Caenorhabditis elegans.Curr Biol. 2000 Sep 21;10(18):1092-7. doi: 10.1016/s0960-9822(00)00691-6. Curr Biol. 2000. PMID: 10996789
-
Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy.Physiol Rev. 2016 Jan;96(1):253-305. doi: 10.1152/physrev.00007.2015. Physiol Rev. 2016. PMID: 26676145 Free PMC article. Review.
Cited by
-
Stress-induced ECM alteration modulates cellular microRNAs that feedback to readjust the extracellular environment and cell behavior.Front Genet. 2013 Dec 31;4:305. doi: 10.3389/fgene.2013.00305. Front Genet. 2013. PMID: 24427166 Free PMC article. Review.
-
Therapeutic aspects of cell signaling and communication in Duchenne muscular dystrophy.Cell Mol Life Sci. 2021 Jun;78(11):4867-4891. doi: 10.1007/s00018-021-03821-x. Epub 2021 Apr 7. Cell Mol Life Sci. 2021. PMID: 33825942 Free PMC article. Review.
-
Key concepts in muscle regeneration: muscle "cellular ecology" integrates a gestalt of cellular cross-talk, motility, and activity to remodel structure and restore function.Eur J Appl Physiol. 2022 Feb;122(2):273-300. doi: 10.1007/s00421-021-04865-4. Epub 2021 Dec 20. Eur J Appl Physiol. 2022. PMID: 34928395 Free PMC article. Review.
-
miRNA-based buffering of the cobblestone-lissencephaly-associated extracellular matrix receptor dystroglycan via its alternative 3'-UTR.Nat Commun. 2014 Sep 18;5:4906. doi: 10.1038/ncomms5906. Nat Commun. 2014. PMID: 25232965 Free PMC article.
-
Non-coding RNAs in muscle dystrophies.Int J Mol Sci. 2013 Sep 30;14(10):19681-704. doi: 10.3390/ijms141019681. Int J Mol Sci. 2013. PMID: 24084719 Free PMC article. Review.
References
-
- Arnold C. P., Tan R., Zhou B., Yue S. B., Schaffert S., Biggs J. R., Doyonnas R., Lo M. C., Perry J. M., Renault V. M., Sacco A., Somervaille T., Viatour P., Brunet A., Cleary M. L., Li L., Sage J., Zhang D. E., Blau H. M., Chen C., Chen C. Z. (2011). MicroRNA programs in normal and aberrant stem and progenitor cells. Genome Res. 21, 798–81010.1101/gr.111385.110 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

