Microsomal glutathione transferase 1 protects against toxicity induced by silica nanoparticles but not by zinc oxide nanoparticles
- PMID: 22303956
- PMCID: PMC3314313
- DOI: 10.1021/nn2021056
Microsomal glutathione transferase 1 protects against toxicity induced by silica nanoparticles but not by zinc oxide nanoparticles
Abstract
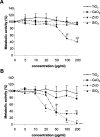
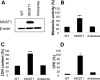
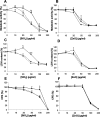
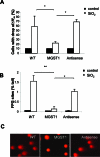
Microsomal glutathione transferase 1 (MGST1) is an antioxidant enzyme located predominantly in the mitochondrial outer membrane and endoplasmic reticulum and has been shown to protect cells from lipid peroxidation induced by a variety of cytostatic drugs and pro-oxidant stimuli. We hypothesized that MGST1 may also protect against nanomaterial-induced cytotoxicity through a specific effect on lipid peroxidation. We evaluated the induction of cytotoxicity and oxidative stress by TiO(2), CeO(2), SiO(2), and ZnO in the human MCF-7 cell line with or without overexpression of MGST1. SiO(2) and ZnO nanoparticles caused dose- and time-dependent toxicity, whereas no obvious cytotoxic effects were induced by nanoparticles of TiO(2) and CeO(2). We also noted pronounced cytotoxicity for three out of four additional SiO(2) nanoparticles tested. Overexpression of MGST1 reversed the cytotoxicity of the main SiO(2) nanoparticles tested and for one of the supplementary SiO(2) nanoparticles but did not protect cells against ZnO-induced cytotoxic effects. The data point toward a role of lipid peroxidation in SiO(2) nanoparticle-induced cell death. For ZnO nanoparticles, rapid dissolution was observed, and the subsequent interaction of Zn(2+) with cellular targets is likely to contribute to the cytotoxic effects. A direct inhibition of MGST1 by Zn(2+) could provide a possible explanation for the lack of protection against ZnO nanoparticles in this model. Our data also showed that SiO(2) nanoparticle-induced cytotoxicity is mitigated in the presence of serum, potentially through masking of reactive surface groups by serum proteins, whereas ZnO nanoparticles were cytotoxic both in the presence and in the absence of serum.
© 2012 American Chemical Society
Figures



Similar articles
-
Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties.ACS Nano. 2008 Oct 28;2(10):2121-34. doi: 10.1021/nn800511k. ACS Nano. 2008. PMID: 19206459 Free PMC article.
-
In vitro evaluation of cellular responses induced by ZnO nanoparticles, zinc ions and bulk ZnO in fish cells.Sci Total Environ. 2013 May 1;452-453:262-74. doi: 10.1016/j.scitotenv.2013.02.079. Epub 2013 Mar 22. Sci Total Environ. 2013. PMID: 23523724
-
Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis.Ecotoxicol Environ Saf. 2015 Mar;113:23-30. doi: 10.1016/j.ecoenv.2014.11.015. Epub 2014 Dec 5. Ecotoxicol Environ Saf. 2015. PMID: 25483368
-
Biological reactivity of zinc oxide nanoparticles with mammalian test systems: an overview.Nanomedicine (Lond). 2015;10(13):2075-92. doi: 10.2217/nnm.15.44. Epub 2015 Jul 2. Nanomedicine (Lond). 2015. PMID: 26135328 Review.
-
Role of MGST1 in reactive intermediate-induced injury.World J Gastroenterol. 2011 May 28;17(20):2552-7. doi: 10.3748/wjg.v17.i20.2552. World J Gastroenterol. 2011. PMID: 21633660 Free PMC article. Review.
Cited by
-
Antibacterial Activity of Dissolved Silver Fractions Released from Silver-Coated Titanium Dental Implant Abutments: A Study on Streptococcus mutans Biofilm Formation.Antibiotics (Basel). 2023 Jun 24;12(7):1097. doi: 10.3390/antibiotics12071097. Antibiotics (Basel). 2023. PMID: 37508193 Free PMC article.
-
Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors.Sci Rep. 2018 Jan 18;8(1):1115. doi: 10.1038/s41598-018-19521-9. Sci Rep. 2018. PMID: 29348435 Free PMC article.
-
Protective mechanisms of a microbial oil against hypercholesterolemia: evidence from a zebrafish model.Front Nutr. 2023 Jun 26;10:1161119. doi: 10.3389/fnut.2023.1161119. eCollection 2023. Front Nutr. 2023. PMID: 37435570 Free PMC article.
-
Exploiting Mass Spectrometry to Unlock the Mechanism of Nanoparticle-Induced Inflammasome Activation.ACS Nano. 2023 Sep 12;17(17):17451-17467. doi: 10.1021/acsnano.3c05600. Epub 2023 Aug 29. ACS Nano. 2023. PMID: 37643371 Free PMC article.
-
Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles.Curr Drug Metab. 2013 Nov;14(9):976-88. doi: 10.2174/1389200211314090004. Curr Drug Metab. 2013. PMID: 24160294 Free PMC article. Review.
References
-
- Auffan M.; Rose J.; Bottero J. Y.; Lowry G. V.; Jolivet J. P.; Wiesner M. R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. - PubMed
-
- Xia T.; Kovochich M.; Brant J.; Hotze M.; Sempf J.; Oberley T.; Sioutas C.; Yeh J. I.; Wiesner M. R.; Nel A. E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. - PubMed
-
- Meng H.; Xia T.; George S.; Nel A. E. A Predictive Toxicological Paradigm for the Safety Assessment of Nanomaterials. ACS Nano 2009, 3, 1620–1627. - PubMed
-
- Shvedova A. A.; Kisin E.; Murray A. R.; Johnson V. J.; Gorelik O.; Arepalli S.; Hubbs A. F.; Mercer R. R.; Keohavong P.; Sussman N.; et al. Inhalation vs. Aspiration of Single-Walled Carbon Nanotubes in C57BL/6 Mice: Inflammation, Fibrosis, Oxidative Stress, and Mutagenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, 552–565. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

