Reaction pathway and free energy profiles for butyrylcholinesterase-catalyzed hydrolysis of acetylthiocholine
- PMID: 22304234
- PMCID: PMC3292049
- DOI: 10.1021/bi201786s
Reaction pathway and free energy profiles for butyrylcholinesterase-catalyzed hydrolysis of acetylthiocholine
Abstract
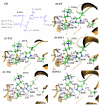
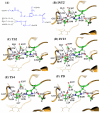
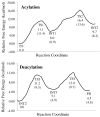
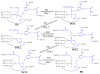
The catalytic mechanism for butyrylcholineserase (BChE)-catalyzed hydrolysis of acetylthiocholine (ATCh) has been studied by performing pseudobond first-principles quantum mechanical/molecular mechanical-free energy (QM/MM-FE) calculations on both acylation and deacylation of BChE. Additional quantum mechanical (QM) calculations have been carried out, along with the QM/MM-FE calculations, to understand the known substrate activation effect on the enzymatic hydrolysis of ATCh. It has been shown that the acylation of BChE with ATCh consists of two reaction steps including the nucleophilic attack on the carbonyl carbon of ATCh and the dissociation of thiocholine ester. The deacylation stage includes nucleophilic attack of a water molecule on the carboxyl carbon of substrate and dissociation between the carboxyl carbon of substrate and hydroxyl oxygen of Ser198 side chain. QM/MM-FE calculation results reveal that the acylation of BChE is rate-determining. It has also been demonstrated that an additional substrate molecule binding to the peripheral anionic site (PAS) of BChE is responsible for the substrate activation effect. In the presence of this additional substrate molecule at PAS, the calculated free energy barrier for the acylation stage (rate-determining step) is decreased by ~1.7 kcal/mol. All of our computational predictions are consistent with available experimental kinetic data. The overall free energy barriers calculated for BChE-catalyzed hydrolysis of ATCh at regular hydrolysis phase and substrate activation phase are ~13.6 and ~11.9 kcal/mol, respectively, which are in reasonable agreement with the corresponding experimentally derived activation free energies of 14.0 kcal/mol (for regular hydrolysis phase) and 13.5 kcal/mol (for substrate activation phase).
Figures

References
-
- Fuxreiter M, Warshel A. Origin of the catalytic power of acetylcholinesterase: Computer simulation studies. J. Am. Chem. Soc. 1998;120:183–194.
-
- Boeck AT, Schopfer LM, Lockridge O. DNA sequence of butyrylcholinesterase from the rat: expression of the protein and characterization of the properties of rat butyrylcholinesterase. Biochem. Pharmacol. 2002;63:2101–2110. - PubMed
-
- Masson P, Froment MT, Gillon E, Nachon F, Lockridge O, Schopfer LM. Kinetic analysis of effector modulation of butyrylcholinesterase-catalysed hydrolysis of acetanilides and homologous esters. FEBS J. 2008;275:2617–2631. - PubMed
-
- Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110:627–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

