A logistic model for the detection of circulating tumour cells in human metastatic colorectal cancer
- PMID: 22304365
- PMCID: PMC3823427
- DOI: 10.1111/j.1582-4934.2012.01544.x
A logistic model for the detection of circulating tumour cells in human metastatic colorectal cancer
Abstract
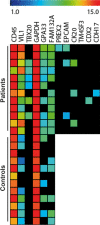
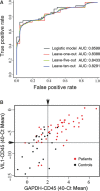
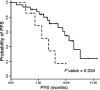
The accuracy in the diagnosis of metastatic colorectal cancer (mCRC) represents one of the challenges in the clinical management of patients. The detection of circulating tumour cells (CTC) is becoming a promising alternative to current detection techniques, as it focuses on one of the players of the metastatic disease and it should provide with more specific and sensitive detection rates. Here, we describe an improved method of detection of CTC from mCRC patients by combining immune-enrichment, optimal purification of RNA from very low cell numbers, and the selection of accurate PCR probes. As a result, we obtained a logistic model that combines GAPDH and VIL1 normalized to CD45 rendering powerful results in the detection of CTC from mCRC patients (AUROC value 0.8599). We further demonstrated the utility of this model at the clinical setting, as a reliable prognosis tool to determine progression-free survival in mCRC patients. Overall, we developed a strategy that ameliorates the specificity and sensitivity in the detection of CTC, resulting in a robust and promising logistic model for the clinical management of metastatic colorectal cancer patients.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

Similar articles
-
A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer.Int J Cancer. 2014 Dec 1;135(11):2633-43. doi: 10.1002/ijc.28910. Epub 2014 Apr 29. Int J Cancer. 2014. PMID: 24752533
-
Cytokeratin-20 and Survivin-Expressing Circulating Tumor Cells Predict Survival in Metastatic Colorectal Cancer Patients by a Combined Immunomagnetic qRT-PCR Approach.Mol Cancer Ther. 2015 Oct;14(10):2401-8. doi: 10.1158/1535-7163.MCT-15-0359. Epub 2015 Jul 30. Mol Cancer Ther. 2015. PMID: 26227487 Free PMC article.
-
Molecular characterization of circulating tumor cells in human metastatic colorectal cancer.PLoS One. 2012;7(7):e40476. doi: 10.1371/journal.pone.0040476. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22811761 Free PMC article.
-
The prognostic role of circulating tumor cells in colorectal cancer.Expert Rev Anticancer Ther. 2019 Dec;19(12):1077-1088. doi: 10.1080/14737140.2019.1699065. Epub 2019 Dec 6. Expert Rev Anticancer Ther. 2019. PMID: 31778322 Review.
-
Detection and Clinical Significance of Circulating Tumor Cells in Colorectal Cancer--20 Years of Progress.Mol Med. 2015 Oct 27;21 Suppl 1(Suppl 1):S25-31. doi: 10.2119/molmed.2015.00149. Mol Med. 2015. PMID: 26605644 Free PMC article. Review.
Cited by
-
Predicting Outcome and Therapy Response in mCRC Patients Using an Indirect Method for CTCs Detection by a Multigene Expression Panel: A Multicentric Prospective Validation Study.Int J Mol Sci. 2017 Jun 13;18(6):1265. doi: 10.3390/ijms18061265. Int J Mol Sci. 2017. PMID: 28608814 Free PMC article.
-
Molecular Profiling of Circulating Tumour Cells Identifies Notch1 as a Principal Regulator in Advanced Non-Small Cell Lung Cancer.Sci Rep. 2016 Nov 30;6:37820. doi: 10.1038/srep37820. Sci Rep. 2016. PMID: 27901069 Free PMC article.
-
Low expression of Talin1 is associated with advanced pathological features in colorectal cancer patients.Sci Rep. 2020 Oct 20;10(1):17786. doi: 10.1038/s41598-020-74810-6. Sci Rep. 2020. PMID: 33082414 Free PMC article.
-
Common molecular markers between circulating tumor cells and blood exosomes in colorectal cancer: a systematic and analytical review.Cancer Manag Res. 2019 Sep 25;11:8669-8698. doi: 10.2147/CMAR.S219699. eCollection 2019. Cancer Manag Res. 2019. PMID: 31576171 Free PMC article.
-
Improving circulating tumor cells enumeration and characterization to predict outcome in first line chemotherapy mCRPC patients.Oncotarget. 2017 May 19;8(33):54708-54721. doi: 10.18632/oncotarget.18025. eCollection 2017 Aug 15. Oncotarget. 2017. PMID: 28903376 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Negin BP, Cohen SJ. Circulating tumour cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010;11:1–13. - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumour cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–9. - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumour cells to tumour response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. - PubMed
-
- Garrigos N, Gallego J, Guillen-Ponce C, et al. Circulating tumour cell analysis as an early marker for relapse in stage II and III colorectal cancer patients: a pilot study. Clin Transl Oncol. 2010;12:142–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

