N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients
- PMID: 22304416
- PMCID: PMC3321095
- DOI: 10.1021/pr201070k
N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients
Abstract
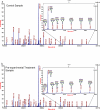
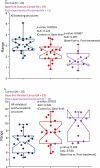
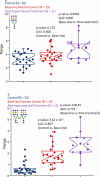
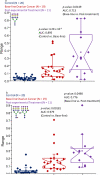
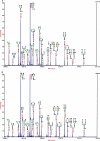
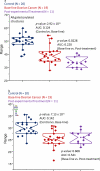
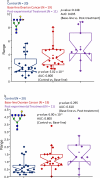
Glycosylated proteins play important roles in a broad spectrum of biochemical and biological processes, and prior reports have suggested that changes in protein glycosylation occur during cancer initiation and progression. Ovarian cancer (OC) is a fatal malignancy, most commonly diagnosed after the development of metastases. Therefore, early detection of OC is key to improving survival. To this end, specific changes of the serum glycome have been proposed as possible biomarkers for different types of cancers. In this study, we extend this concept to OC. To characterize differences in total N-glycan levels, serum samples provided by 20 healthy control women were compared to those acquired from patients diagnosed with late-stage recurrent OC who were enrolled in an experimental treatment trial prior to receiving therapy (N=19) and one month later, prior to the second treatment cycle (N=11). Additionally, analyses of the N-glycans associated with IgG and characterization of the relative abundance levels of core vs outer-arm fucosylation were also performed. The N-linked glycomic profiles revealed increased abundances of tri- and tetra-branched structures with varying degrees of sialylation and fucosylation and an apparent decrease in the levels of "bisecting" glycans in OC samples compared to controls. Increased levels of a-galactosylation structures were observed on N-linked glycans derived from IgG, which were independent of the presence of fucose residues. Elevated levels of outer-arm fucosylation were also identified in the OC samples. These results allowed the control samples to be distinguished from the baseline ovarian cancer patients prior to receiving the experimental treatment. In some cases, the pre-treatment samples could be distinguished from the post-experimental treatment samples, as many of those patients showed a further progression of the disease.
Figures





References
-
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–88. - PubMed
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. - PubMed
-
- Arnold JN, Saldova R, Hamid UM, Rudd PM. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008;8:3284–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

