Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities
- PMID: 22304503
- PMCID: PMC3353819
- DOI: 10.1089/ars.2011.4198
Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities
Abstract
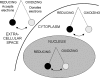
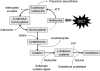
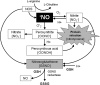
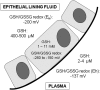
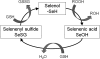
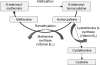
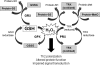
Asthma is a chronic inflammatory disorder of the airways associated with airway hyper-responsiveness and airflow limitation in response to specific triggers. Whereas inflammation is important for tissue regeneration and wound healing, the profound and sustained inflammatory response associated with asthma may result in airway remodeling that involves smooth muscle hypertrophy, epithelial goblet-cell hyperplasia, and permanent deposition of airway extracellular matrix proteins. Although the specific mechanisms responsible for asthma are still being unraveled, free radicals such as reactive oxygen species and reactive nitrogen species are important mediators of airway tissue damage that are increased in subjects with asthma. There is also a growing body of literature implicating disturbances in oxidation/reduction (redox) reactions and impaired antioxidant defenses as a risk factor for asthma development and asthma severity. Ultimately, these redox-related perturbations result in a vicious cycle of airway inflammation and injury that is not always amenable to current asthma therapy, particularly in cases of severe asthma. This review will discuss disruptions of redox signaling and control in asthma with a focus on the thiol, glutathione, and reduced (thiol) form (GSH). First, GSH synthesis, GSH distribution, and GSH function and homeostasis are discussed. We then review the literature related to GSH redox balance in health and asthma, with an emphasis on human studies. Finally, therapeutic opportunities to restore the GSH redox balance in subjects with asthma are discussed.
Figures
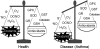








References
-
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. - PubMed
-
- Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. - PubMed
-
- Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

