Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters
- PMID: 22304918
- PMCID: PMC3292199
- DOI: 10.1016/j.cell.2012.01.007
Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters
Abstract
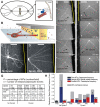
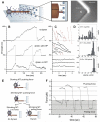
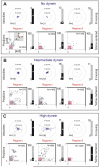
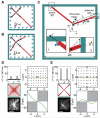
Dynein at the cortex contributes to microtubule-based positioning processes such as spindle positioning during embryonic cell division and centrosome positioning during fibroblast migration. To investigate how cortical dynein interacts with microtubule ends to generate force and how this functional association impacts positioning, we have reconstituted the 'cortical' interaction between dynein and dynamic microtubule ends in an in vitro system using microfabricated barriers. We show that barrier-attached dynein captures microtubule ends, inhibits growth, and triggers microtubule catastrophes, thereby controlling microtubule length. The subsequent interaction with shrinking microtubule ends generates pulling forces up to several pN. By combining experiments in microchambers with a theoretical description of aster mechanics, we show that dynein-mediated pulling forces lead to the reliable centering of microtubule asters in simple confining geometries. Our results demonstrate the intrinsic ability of cortical microtubule-dynein interactions to regulate microtubule dynamics and drive positioning processes in living cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Chang F, Nurse P. How fission yeast fission in the middle. Cell. 1996;84:191–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

