A dynamic network of morphogens and transcription factors patterns the fly leg
- PMID: 22305163
- PMCID: PMC3918458
- DOI: 10.1016/B978-0-12-386499-4.00007-0
A dynamic network of morphogens and transcription factors patterns the fly leg
Abstract
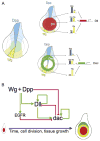
Animal appendages require a proximodistal (PD) axis, which forms orthogonally from the two main body axes, anteroposterior and dorsoventral. In this review, we discuss recent advances that begin to provide insights into the molecular mechanisms controlling PD axis formation in the Drosophila leg. In this case, two morphogens, Wingless (Wg) and Decapentaplegic (Dpp), initiate a genetic cascade that, together with growth of the leg imaginal disc, establishes the PD axis. The analysis of cis-regulatory modules (CRMs) that control the expression of genes at different positions along the PD axis has been particularly valuable in dissecting this complex process. From these experiments, it appears that only one concentration of Wg and Dpp are required to initiate PD axis formation by inducing the expression of Distal-less (Dll), a homeodomain-encoding gene that is required for leg development. Once Dll is turned on, it activates the medially expressed gene dachshund (dac). Cross-regulation between Dll and dac, together with cell proliferation in the growing leg imaginal disc, results in the formation of a rudimentary PD axis. Wg and Dpp also initiate the expression of ligands for the EGFR pathway, which in turn induces the expression of a series of target genes that pattern the distal-most portion of the leg.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
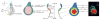
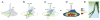



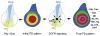
References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
-
- Azpiazu N, Morata G. Distinct functions of homothorax in leg development in Drosophila. Mech Dev. 2002;119:55–67. - PubMed
-
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

