Identification of avian wax synthases
- PMID: 22305293
- PMCID: PMC3316144
- DOI: 10.1186/1471-2091-13-4
Identification of avian wax synthases
Abstract
Background: Bird species show a high degree of variation in the composition of their preen gland waxes. For instance, galliform birds like chicken contain fatty acid esters of 2,3-alkanediols, while Anseriformes like goose or Strigiformes like barn owl contain wax monoesters in their preen gland secretions. The final biosynthetic step is catalyzed by wax synthases (WS) which have been identified in pro- and eukaryotic organisms.
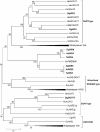
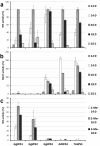
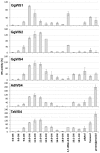
Results: Sequence similarities enabled us to identify six cDNAs encoding putative wax synthesizing proteins in chicken and two from barn owl and goose. Expression studies in yeast under in vivo and in vitro conditions showed that three proteins from chicken performed WS activity while a sequence from chicken, goose and barn owl encoded a bifunctional enzyme catalyzing both wax ester and triacylglycerol synthesis. Mono- and bifunctional WS were found to differ in their substrate specificities especially with regard to branched-chain alcohols and acyl-CoA thioesters. According to the expression patterns of their transcripts and the properties of the enzymes, avian WS proteins might not be confined to preen glands.
Conclusions: We provide direct evidence that avian preen glands possess both monofunctional and bifunctional WS proteins which have different expression patterns and WS activities with different substrate specificities.
Figures




References
-
- Jacob J, Balthazart J, Schoffeniels E. Sex Differences in the Chemical Composition of Uropygial Gland Waxes in Domestic Ducks. Biochemical Systematics and Ecology. 1979;7:149–153. doi: 10.1016/0305-1978(79)90024-3. - DOI
-
- Piersma T, Dekker M, Damste JSS. An Avian Equivalent of Make-Up? Ecol Lett. 1999;2:201–203. doi: 10.1046/j.1461-0248.1999.00078.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

