A strategy of gene overexpression based on tandem repetitive promoters in Escherichia coli
- PMID: 22305426
- PMCID: PMC3293061
- DOI: 10.1186/1475-2859-11-19
A strategy of gene overexpression based on tandem repetitive promoters in Escherichia coli
Abstract
Background: For metabolic engineering, many rate-limiting steps may exist in the pathways of accumulating the target metabolites. Increasing copy number of the desired genes in these pathways is a general method to solve the problem, for example, the employment of the multi-copy plasmid-based expression system. However, this method may bring genetic instability, structural instability and metabolic burden to the host, while integrating of the desired gene into the chromosome may cause inadequate transcription or expression. In this study, we developed a strategy for obtaining gene overexpression by engineering promoter clusters consisted of multiple core-tac-promoters (MCPtacs) in tandem.
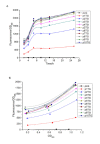
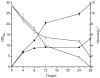
Results: Through a uniquely designed in vitro assembling process, a series of promoter clusters were constructed. The transcription strength of these promoter clusters showed a stepwise enhancement with the increase of tandem repeats number until it reached the critical value of five. Application of the MCPtacs promoter clusters in polyhydroxybutyrate (PHB) production proved that it was efficient. Integration of the phaCAB genes with the 5CPtacs promoter cluster resulted in an engineered E.coli that can accumulate 23.7% PHB of the cell dry weight in batch cultivation.
Conclusions: The transcription strength of the MCPtacs promoter cluster can be greatly improved by increasing the tandem repeats number of the core-tac-promoter. By integrating the desired gene together with the MCPtacs promoter cluster into the chromosome of E. coli, we can achieve high and stale overexpression with only a small size. This strategy has an application potential in many fields and can be extended to other bacteria.
Figures


Similar articles
-
Enhanced polyhydroxybutyrate (PHB) production via the coexpressed phaCAB and vgb genes controlled by arabinose P promoter in Escherichia coli.Lett Appl Microbiol. 2010 Feb;50(2):158-67. doi: 10.1111/j.1472-765X.2009.02772.x. Epub 2009 Nov 12. Lett Appl Microbiol. 2010. PMID: 19943886
-
Effects of cascaded vgb promoters on poly(hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli grown micro-aerobically.Appl Microbiol Biotechnol. 2014 Dec;98(24):10013-21. doi: 10.1007/s00253-014-6059-y. Epub 2014 Sep 13. Appl Microbiol Biotechnol. 2014. PMID: 25216582
-
[Efficient polyhydroxybutyrate production from cheap resources by recombinant Escherichia coli].Sheng Wu Gong Cheng Xue Bao. 2010 Sep;26(9):1257-62. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 21141116 Chinese.
-
A novel strategy for succinate and polyhydroxybutyrate co-production in Escherichia coli.Bioresour Technol. 2010 Oct;101(19):7675-8. doi: 10.1016/j.biortech.2010.04.084. Epub 2010 May 15. Bioresour Technol. 2010. PMID: 20472425
-
Expression of highly toxic genes in E. coli: special strategies and genetic tools.Curr Protein Pept Sci. 2006 Feb;7(1):47-56. doi: 10.2174/138920306775474095. Curr Protein Pept Sci. 2006. PMID: 16472168 Review.
Cited by
-
One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of L-tryptophan in Escherichia coli.Microb Cell Fact. 2012 Mar 2;11:30. doi: 10.1186/1475-2859-11-30. Microb Cell Fact. 2012. PMID: 22380540 Free PMC article.
-
Synthetic sugar cassettes for the efficient production of flavonol glycosides in Escherichia coli.Microb Cell Fact. 2015 Jun 9;14:76. doi: 10.1186/s12934-015-0261-1. Microb Cell Fact. 2015. PMID: 26051114 Free PMC article.
-
Rhodaneses minimize the accumulation of cellular sulfane sulfur to avoid disulfide stress during sulfide oxidation in bacteria.Redox Biol. 2022 Jul;53:102345. doi: 10.1016/j.redox.2022.102345. Epub 2022 May 26. Redox Biol. 2022. PMID: 35653932 Free PMC article.
-
An insight into the "-omics" based engineering of streptomycetes for secondary metabolite overproduction.Biomed Res Int. 2013;2013:968518. doi: 10.1155/2013/968518. Epub 2013 Sep 2. Biomed Res Int. 2013. PMID: 24078931 Free PMC article. Review.
-
T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis.Nucleic Acids Res. 2019 Feb 20;47(3):e15. doi: 10.1093/nar/gky1169. Nucleic Acids Res. 2019. PMID: 30462336 Free PMC article.
References
-
- Varma A, Palsson BO. Metabolic flux balancing: basic concepts, scientific and practical use. Nat Biotechnol. 1994;12(10):994–998. doi: 10.1038/nbt1094-994. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

