The phenotype of a germline mutation in PIGA: the gene somatically mutated in paroxysmal nocturnal hemoglobinuria
- PMID: 22305531
- PMCID: PMC3276655
- DOI: 10.1016/j.ajhg.2011.11.031
The phenotype of a germline mutation in PIGA: the gene somatically mutated in paroxysmal nocturnal hemoglobinuria
Abstract
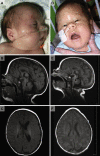
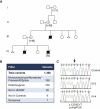
Phosphatidylinositol glycan class A (PIGA) is involved in the first step of glycosylphosphatidylinositol (GPI) biosynthesis. Many proteins, including CD55 and CD59, are anchored to the cell by GPI. Loss of CD55 and CD59 on erythrocytes causes complement-mediated lysis in paroxysmal nocturnal hemoglobinuria (PNH), a disease that manifests after clonal expansion of hematopoietic cells with somatic PIGA mutations. Although somatic PIGA mutations have been identified in many PNH patients, it has been proposed that germline mutations are lethal. We report a family with an X-linked lethal disorder involving cleft palate, neonatal seizures, contractures, central nervous system (CNS) structural malformations, and other anomalies. An X chromosome exome next-generation sequencing screen identified a single nonsense PIGA mutation, c.1234C>T, which predicts p.Arg412(∗). This variant segregated with disease and carrier status in the family, is similar to mutations known to cause PNH as a result of PIGA dysfunction, and was absent in 409 controls. PIGA-null mutations are thought to be embryonic lethal, suggesting that p.Arg412(∗) PIGA has residual function. Transfection of a mutant p.Arg412(∗) PIGA construct into PIGA-null cells showed partial restoration of GPI-anchored proteins. The genetic data show that the c.1234C>T (p.Arg412(∗)) mutation is present in an affected child, is linked to the affected chromosome in this family, is rare in the population, and results in reduced, but not absent, biosynthesis of GPI anchors. We conclude that c.1234C>T in PIGA results in the lethal X-linked phenotype recognized in the reported family.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Almeida A., Layton M., Karadimitris A. Inherited glycosylphosphatidyl inositol deficiency: A treatable CDG. Biochim. Biophys. Acta. 2009;1792:874–880. - PubMed
-
- Brodsky R.A. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann. Intern. Med. 2008;148:587–595. - PubMed
-
- Ware R.E., Rosse W.F., Howard T.A. Mutations within the Piga gene in patients with paroxysmal nocturnal hemoglobinuria. Blood. 1994;83:2418–2422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

