The potential of stem cells as an in vitro source of red blood cells for transfusion
- PMID: 22305561
- PMCID: PMC3676433
- DOI: 10.1016/j.stem.2012.01.001
The potential of stem cells as an in vitro source of red blood cells for transfusion
Abstract
Recent advances have increased excitement about the potential for therapeutic production of red blood cells (RBCs) in vitro. However, generation of RBCs in the large numbers required for transfusion remains a significant challenge. In this article, we summarize recent progress in producing RBCs from various cell sources, and discuss the hurdles that remain for translation into the clinical arena.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
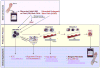
References
-
- Chang AH, Stephan MT, Sadelain M. Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol. 2006a;24:1017–1021. - PubMed
-
- Chaurasia P, Berenzon D, Hoffman R. Chromatin-modifying agents promote the ex vivo production of functional human erythroid progenitor cells. Blood. 2011;117:4632–4641. - PubMed
WEB RESOURCES
-
- American Society of Hematology. Press release: Researchers Successfully Perform First Injection of Cultured Red Blood Cells in Human Donor. 2011 Sep 1; 2011. ( http://www.hematology.org/News/2011/6995.aspx)
-
- European Medicines Agency. 2011 http://www.ema.europa.eu.
-
- LifeShare Blood Centers. Blood Facts Report. 2011 ( http://www.lifeshare.org/facts/raretraits.htm)
-
- US Department of Health and Human Services. Clinical Trials. Gov: Cultured Red Blood Cells: Life Span in-vivo Studies (GRc2008) 2008 ( http://www.clinicaltrials.gov/ct2/show/NCT00929266)
-
- US Department of Health and Human Services. American Association of Blood Banks 2009 National Blood Collection and Utilization Report. 2009 ( http://www.aabb.org/programs/biovigilance/nbcus/Documents/09-nbcus-repor...)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

