Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis
- PMID: 22305802
- PMCID: PMC4063417
- DOI: 10.1016/S1474-4422(12)70015-7
Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis
Abstract
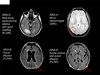
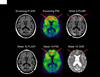
Background: Amyloid-related imaging abnormalities (ARIA) have been reported in patients with Alzheimer's disease treated with bapineuzumab, a humanised monoclonal antibody against amyloid β. ARIA include MRI signal abnormalities suggestive of vasogenic oedema and sulcal effusions (ARIA-E) and microhaemorrhages and haemosiderin deposits (ARIA-H). Our aim was to investigate the incidence of ARIA during treatment with bapineuzumab, and evaluate associated risk factors.
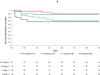
Methods: Two neuroradiologists independently reviewed 2572 fluid-attenuated inversion recovery (FLAIR) MRI scans from 262 participants in two phase 2 studies of bapineuzumab and an open-label extension study. Readers were masked to the patient's treatment, APOE ɛ4 genotype, medical history, and demographics. Patients were included in risk analyses if they had no evidence of ARIA-E in their pre-treatment MRI, had received bapineuzumab, and had at least one MRI scan after treatment. We used Kaplan-Meier survival analysis to examine the distribution of incident ARIA-E from the start of bapineuzumab treatment and proportional hazards regression models to assess risk factors associated with ARIA.
Findings: 210 patients were included in the risk analyses. 36 patients (17%) developed ARIA-E during treatment with bapineuzumab; 15 of these ARIA-E cases (42%) had not been detected previously. 28 of these patients (78%) did not report associated symptoms. Adverse events, reported in eight symptomatic patients, included headache, confusion, and neuropsychiatric and gastrointestinal symptoms. Incident ARIA-H occurred in 17 of the patients with ARIA-E (47%), compared with seven of 177 (4%) patients without ARIA-E. 13 of the 15 patients in whom ARIA were detected in our study received additional treatment infusions while ARIA-E were present, without any associated symptoms. Occurrence of ARIA-E increased with bapineuzumab dose (hazard ratio [HR] 2·24 per 1 mg/kg increase in dose, 95% CI 1·40-3·62; p=0·0008) and presence of APOE ɛ4 alleles (HR 2·55 per allele, 95% CI 1·57-4·12; p=0·0001).
Interpretation: ARIA consist of a spectrum of imaging findings with variable clinical correlates, and some patients with ARIA-E remain asymptomatic even if treatment is continued. The increased risk of ARIA among APOE ɛ4 carriers, its association with high bapineuzumab dose, and its timecourse in relation to dosing suggest an association between ARIA and alterations in vascular amyloid burden.
Funding: Elan Corporation, Janssen Alzheimer Immunotherapy, Wyeth Pharmaceuticals, and Pfizer.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
RS has served as a study investigator and a consultant for Janssen Alzheimer Immunotherapy Research & Development, LLC, and for Pfizer Inc., and has received honoraria for participation in symposiums. She has also served as a consultant and/or site investigator for Bristol-Myers-Squibb, Roche, Elan, Biogen-IDEC, Avid, and Bayer. SS has served as a consultant and study investigator for Janssen Alzheimer Immunotherapy Research & Development, LLC., Pfizer Inc., and Elan Corporation, plc. phase 2 and 3 studies of bapineuzumab. DB reports no conflicts of interest. DT provides review of MRI images for Janssen Alzheimer Immunotherapy Research & Development, LLC. JB serves as a neuroradiological consultant to SYNARC Inc., an imaging contract research organization contracted by both sponsor companies (Janssen Alzheimer Immunotherapy Research & Development, LLC. and Pfizer Inc.); he also serves as a consultant to Janssen Alzheimer Immunotherapy Research & Development, LLC. for non-clinical research activities. NF has provided consulting and/or image analysis services to Elan Corporation, plc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Pfizer Inc., and Wyeth Pharmaceuticals as well as to AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GE Healthcare, Lundbeck A/S, and IXICO. MR serves as consultant to Janssen Alzheimer Immunotherapy Research & Development, LLC. MS participates in a consulting/advisory capacity for Eli Lilly and Company, Amerisciences, Takeda Pharmaceuticals Inc., Eisai Co., Ltd., Pfizer Inc., and GlaxoSmithKline plc. and receives royalties from Wiley and AmeriSciences, LP. He receives contracting fees/grants from Celgene Corporation, Ceregene, Inc., Bayer AG, Baxter International Inc., Bristol-Myers Squibb, Eli Lilly and Company, Pfizer Inc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Avid Radiopharmaceuticals, Inc., Genentech, Inc. and Eisai Co., Ltd. LH serves on the study steering committee and has acted as a consultant for Janssen Alzheimer Immunotherapy Research & Development, LLC., but receives less than $10,000 annually for such consulting activities. AP has received grant/research support from Baxter International Inc., Bristol-Myers Squibb, Eisai Co., Ltd., Elan Corporation, plc., Genentech, Inc./ Hoffmann-La Roche Inc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Medivation, Inc., Pfizer Inc., and Toyama Chemical Co., Ltd. He has also served as a consultant/participated on advisory boards for Elan Corporation, plc., Janssen Alzheimer Immunotherapy Research & Development, LLC., Medivation, Inc., Pfizer Inc., Transition Therapeutics Inc., and Toyama Chemical Co., Ltd.. He is also a member of the speakers’ bureau for Forest Laboratories, Inc. IL is a stockholder in Elan Corporation, plc. RB is an employee of and receives stock and stock options from Pfizer Inc. MA, KM, YL, EL, KG, RHB, and GK are employees of Janssen Alzheimer Immunotherapy Research & Development, LLC. MG is a consultant to Janssen Alzheimer Immunotherapy Research & Development, LLC. and is a stockholder in Elan Corporation, plc.
Figures




Comment in
-
ARIA from off-key operas?Lancet Neurol. 2012 Mar;11(3):207-8. doi: 10.1016/S1474-4422(12)70021-2. Lancet Neurol. 2012. PMID: 22341028 No abstract available.
References
-
- Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–72. - PubMed
-
- Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

