A causal framework for understanding the effect of losses to follow-up on epidemiologic analyses in clinic-based cohorts: the case of HIV-infected patients on antiretroviral therapy in Africa
- PMID: 22306557
- PMCID: PMC3353135
- DOI: 10.1093/aje/kwr444
A causal framework for understanding the effect of losses to follow-up on epidemiologic analyses in clinic-based cohorts: the case of HIV-infected patients on antiretroviral therapy in Africa
Abstract
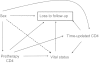
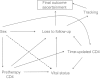
Although clinic-based cohorts are most representative of the "real world," they are susceptible to loss to follow-up. Strategies for managing the impact of loss to follow-up are therefore needed to maximize the value of studies conducted in these cohorts. The authors evaluated adult patients starting antiretroviral therapy at an HIV/AIDS clinic in Uganda, where 29% of patients were lost to follow-up after 2 years (January 1, 2004-September 30, 2007). Unweighted, inverse probability of censoring weighted (IPCW), and sampling-based approaches (using supplemental data from a sample of lost patients subsequently tracked in the community) were used to identify the predictive value of sex on mortality. Directed acyclic graphs (DAGs) were used to explore the structural basis for bias in each approach. Among 3,628 patients, unweighted and IPCW analyses found men to have higher mortality than women, whereas the sampling-based approach did not. DAGs encoding knowledge about the data-generating process, including the fact that death is a cause of being classified as lost to follow-up in this setting, revealed "collider" bias in the unweighted and IPCW approaches. In a clinic-based cohort in Africa, unweighted and IPCW approaches-which rely on the "missing at random" assumption-yielded biased estimates. A sampling-based approach can in general strengthen epidemiologic analyses conducted in many clinic-based cohorts, including those examining other diseases.
Figures
References
-
- Gandhi M, Ameli N, Bacchetti P, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS. 2005;19(16):1885–1896. - PubMed
-
- Lee PY, Alexander KP, Hammill BG, et al. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286(6):708–713. - PubMed
-
- Indiana University. International Epidemiologic Databases to Evaluate AIDS. Indianapolis, IN: Indiana University; 2011. ( http://www.iedea-hiv.org/). (Accessed January 21, 2011)
-
- van Oosterhout JJ, Bodasing N, Kumwenda JJ, et al. Evaluation of antiretroviral therapy results in a resource-poor setting in Blantyre, Malawi. Trop Med Int Health. 2005;10(5):464–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

