The composition and signaling of the IL-35 receptor are unconventional
- PMID: 22306691
- PMCID: PMC3529151
- DOI: 10.1038/ni.2227
The composition and signaling of the IL-35 receptor are unconventional
Abstract
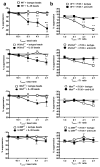
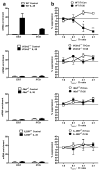
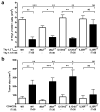
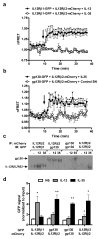
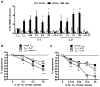
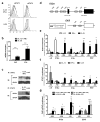
Interleukin 35 (IL-35) belongs to the IL-12 family of heterodimeric cytokines but has a distinct functional profile. IL-35 suppresses T cell proliferation and converts naive T cells into IL-35-producing induced regulatory T cells (iTr35 cells). Here we found that IL-35 signaled through a unique heterodimer of receptor chains IL-12Rβ2 and gp130 or homodimers of each chain. Conventional T cells were sensitive to IL-35-mediated suppression in the absence of one receptor chain but not both receptor chains, whereas signaling through both chains was required for IL-35 expression and conversion into iTr35 cells. Signaling through the IL-35 receptor required the transcription factors STAT1 and STAT4, which formed a unique heterodimer that bound to distinct sites in the promoters of the genes encoding the IL-12 subunits p35 and Ebi3. This unconventional mode of signaling, distinct from that of other members of the IL-12 family, may broaden the spectrum and specificity of IL-35-mediated suppression.
Conflict of interest statement
The authors declare competing financial interests. D.A.A.V., L.W.C. and K.M.V. have submitted patents that are pending and are entitled to a share in net income generated from licensing of these patent rights for commercial development. Furthermore, this work was supported in part by a sponsored research agreement with NovoNordisk to D.A.A.V.
Figures


References
-
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. - PubMed
-
- O’Shea JJ, Paul WE. Regulation of T(H)1 differentiation--controlling the controllers. Nat Immunol. 2002;3:506–508. - PubMed
-
- Artis D, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. - PubMed
-
- Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

