A CD74-dependent MHC class I endolysosomal cross-presentation pathway
- PMID: 22306692
- PMCID: PMC4933585
- DOI: 10.1038/ni.2225
A CD74-dependent MHC class I endolysosomal cross-presentation pathway
Abstract
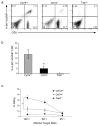
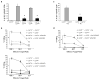
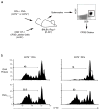
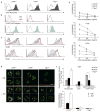
Immune responses are initiated and primed by dendritic cells (DCs) that cross-present exogenous antigen. The chaperone CD74 (invariant chain) is thought to promote DC priming exclusively in the context of major histocompatibility complex (MHC) class II. However, we demonstrate here a CD74-dependent MHC class I cross-presentation pathway in DCs that had a major role in the generation of MHC class I-restricted, cytolytic T lymphocyte (CTL) responses to viral protein- and cell-associated antigens. CD74 associated with MHC class I in the endoplasmic reticulum of DCs and mediated the trafficking of MHC class I to endolysosomal compartments for loading with exogenous peptides. We conclude that CD74 has a previously undiscovered physiological function in endolysosomal DC cross-presentation for priming MHC class I-mediated CTL responses.
Figures
Comment in
-
An invariant road to cross-presentation.Nat Immunol. 2012 Feb 16;13(3):207-8. doi: 10.1038/ni.2235. Nat Immunol. 2012. PMID: 22344275 No abstract available.
References
-
- Guagliardi LE, et al. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133–139. - PubMed
-
- Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. - PubMed
-
- Guermonprez P, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. - PubMed
-
- Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

