Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis
- PMID: 22306733
- PMCID: PMC3998727
- DOI: 10.1038/nm.2629
Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis
Abstract
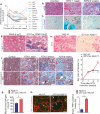
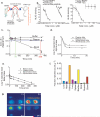
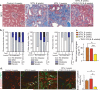
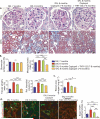
Molecules associated with the transforming growth factor β (TGF-β) superfamily, such as bone morphogenic proteins (BMPs) and TGF-β, are key regulators of inflammation, apoptosis and cellular transitions. Here we show that the BMP receptor activin-like kinase 3 (Alk3) is elevated early in diseased kidneys after injury. We also found that its deletion in the tubular epithelium leads to enhanced TGF-β1-Smad family member 3 (Smad3) signaling, epithelial damage and fibrosis, suggesting a protective role for Alk3-mediated signaling in the kidney. A structure-function analysis of the BMP-Alk3-BMP receptor, type 2 (BMPR2) ligand-receptor complex, along with synthetic organic chemistry, led us to construct a library of small peptide agonists of BMP signaling that function through the Alk3 receptor. One such peptide agonist, THR-123, suppressed inflammation, apoptosis and the epithelial-to-mesenchymal transition program and reversed established fibrosis in five mouse models of acute and chronic renal injury. THR-123 acts specifically through Alk3 signaling, as mice with a targeted deletion for Alk3 in their tubular epithelium did not respond to therapy with THR-123. Combining THR-123 and the angiotensin-converting enzyme inhibitor captopril had an additive therapeutic benefit in controlling renal fibrosis. Our studies show that BMP signaling agonists constitute a new line of therapeutic agents with potential utility in the clinic to induce regeneration, repair and reverse established fibrosis.
Figures

Comment in
-
Basic research: an oral cyclic peptide drug to reverse kidney fibrosis?Nat Rev Nephrol. 2012 Feb 28;8(4):193. doi: 10.1038/nrneph.2012.26. Nat Rev Nephrol. 2012. PMID: 22371249 No abstract available.
-
Regarding the mechanism of action of a proposed peptide agonist of the bone morphogenetic protein receptor activin-like kinase 3.Nat Med. 2013 Jul;19(7):809-10. doi: 10.1038/nm.3080. Nat Med. 2013. PMID: 23836213 No abstract available.
-
Reply to Regarding the mechanism of action of a proposed peptide agonist of the bone morphogenetic protein receptor activin-like kinase 3.Nat Med. 2013 Jul;19(7):810-1. doi: 10.1038/nm.3081. Nat Med. 2013. PMID: 23836214 No abstract available.
References
-
- Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med. 2004;82:175–181. - PubMed
-
- Zeisberg M, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. - PubMed
-
- Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. - PubMed
-
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA163191/CA/NCI NIH HHS/United States
- DK55001/DK/NIDDK NIH HHS/United States
- U01 CA151925/CA/NCI NIH HHS/United States
- DK074558/DK/NIDDK NIH HHS/United States
- CA163191/CA/NCI NIH HHS/United States
- CA151925/CA/NCI NIH HHS/United States
- CA155370/CA/NCI NIH HHS/United States
- T32 HL007374/HL/NHLBI NIH HHS/United States
- 5T32HL007374-30/HL/NHLBI NIH HHS/United States
- DK081576/DK/NIDDK NIH HHS/United States
- T32 DK007760/DK/NIDDK NIH HHS/United States
- R01 DK081576/DK/NIDDK NIH HHS/United States
- R01 DK061688/DK/NIDDK NIH HHS/United States
- DK061688/DK/NIDDK NIH HHS/United States
- K08 DK074558/DK/NIDDK NIH HHS/United States
- 2T32DK007760-11/DK/NIDDK NIH HHS/United States
- R01 CA125550/CA/NCI NIH HHS/United States
- CA125550/CA/NCI NIH HHS/United States
- R01 DK055001/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

