Modulation of the interaction between neurotensin receptor NTS1 and Gq protein by lipid
- PMID: 22306739
- PMCID: PMC3294418
- DOI: 10.1016/j.jmb.2012.01.023
Modulation of the interaction between neurotensin receptor NTS1 and Gq protein by lipid
Abstract
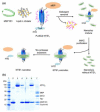
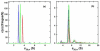
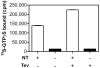
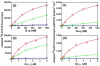
Membrane lipids have been implicated to influence the activity of G-protein-coupled receptors (GPCRs). Almost all of our knowledge on the role of lipids on GPCR and G protein function comes from work on the visual pigment rhodopsin and its G protein transducin, which reside in a highly specialized membrane environment. Thus, insight gained from rhodopsin signaling may not be simply translated to other nonvisual GPCRs. Here, we investigated the effect of lipid head group charges on the signal transduction properties of the class A GPCR neurotensin (NT) receptor 1 (NTS1) under defined experimental conditions, using self-assembled phospholipid nanodiscs prepared with the zwitter-ionic lipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), the negatively charged 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (POPG), or a POPC/POPG mixture. A combination of dynamic light scattering and sedimentation velocity showed that NTS1 was monomeric in POPC-, POPC/POPG-, and POPG-nanodiscs. Binding of the agonist NT to NTS1 occurred with similar affinities and was essentially unaffected by the phospholipid composition. In contrast, Gq protein coupling to NTS1 in various lipid nanodiscs was significantly different, and the apparent affinity of Gαq and Gβ(1)γ(1) to activated NTS1 increased with increasing POPG content. NTS1-catalyzed GDP/GTPγS nucleotide exchange at Gαq in the presence of Gβ(1)γ(1) and NT was crucially affected by the lipid type, with exchange rates higher by 1 or 2 orders of magnitude in POPC/POPG- and POPG-nanodiscs, respectively, compared to POPC-nanodiscs. Our data demonstrate that negatively charged lipids in the immediate vicinity of a nonvisual GPCR modulate the G-protein-coupling step.
Published by Elsevier Ltd.
Figures


Similar articles
-
Lipid modulation of early G protein-coupled receptor signalling events.Biochim Biophys Acta. 2015 Nov;1848(11 Pt A):2889-97. doi: 10.1016/j.bbamem.2015.08.004. Epub 2015 Aug 11. Biochim Biophys Acta. 2015. PMID: 26275588
-
A coarse-grained approach to studying the interactions of the antimicrobial peptides aurein 1.2 and maculatin 1.1 with POPG/POPE lipid mixtures.J Mol Model. 2018 Jul 17;24(8):208. doi: 10.1007/s00894-018-3747-z. J Mol Model. 2018. PMID: 30019106
-
The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants.Biochim Biophys Acta. 2011 Mar;1808(3):622-33. doi: 10.1016/j.bbamem.2010.11.025. Epub 2010 Dec 7. Biochim Biophys Acta. 2011. PMID: 21144817
-
Molecular and cellular regulation of neurotensin receptor under acute and chronic agonist stimulation.Peptides. 2006 Oct;27(10):2493-501. doi: 10.1016/j.peptides.2006.04.029. Epub 2006 Aug 4. Peptides. 2006. PMID: 16889873 Review.
-
Interactions between neurotensin receptors and G proteins.Peptides. 2006 Oct;27(10):2476-87. doi: 10.1016/j.peptides.2006.04.027. Epub 2006 Aug 17. Peptides. 2006. PMID: 16919370 Review.
Cited by
-
Sarcolemmal dependence of cardiac protection and stress-resistance: roles in aged or diseased hearts.Br J Pharmacol. 2016 Oct;173(20):2966-91. doi: 10.1111/bph.13552. Epub 2016 Sep 9. Br J Pharmacol. 2016. PMID: 27439627 Free PMC article. Review.
-
Local membrane charge regulates β2 adrenergic receptor coupling to Gi3.Nat Commun. 2019 May 20;10(1):2234. doi: 10.1038/s41467-019-10108-0. Nat Commun. 2019. PMID: 31110175 Free PMC article.
-
Functional G-Protein-Coupled Receptor (GPCR) Synthesis: The Pharmacological Analysis of Human Histamine H1 Receptor (HRH1) Synthesized by a Wheat Germ Cell-Free Protein Synthesis System Combined with Asolectin Glycerosomes.Front Pharmacol. 2018 Feb 6;9:38. doi: 10.3389/fphar.2018.00038. eCollection 2018. Front Pharmacol. 2018. PMID: 29467651 Free PMC article.
-
Lipid Dynamics in Diisobutylene-Maleic Acid (DIBMA) Lipid Particles in Presence of Sensory Rhodopsin II.Int J Mol Sci. 2021 Mar 4;22(5):2548. doi: 10.3390/ijms22052548. Int J Mol Sci. 2021. PMID: 33806280 Free PMC article.
-
From atomic structures to neuronal functions of g protein-coupled receptors.Annu Rev Neurosci. 2013 Jul 8;36:139-64. doi: 10.1146/annurev-neuro-062012-170313. Epub 2013 May 15. Annu Rev Neurosci. 2013. PMID: 23682660 Free PMC article. Review.
References
-
- Lee AG. Biological membranes: the importance of molecular detail. Trends Biochem. Sci. 2011;36:493–500. - PubMed
-
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th Edition W.H. Freeman and Company; New York: 2002.
-
- Alemany R, Perona JS, Sanchez-Dominguez JM, Montero E, Canizares J, Bressani R, et al. G protein-coupled receptor systems and their lipid environment in health disorders during aging. Biochim. Biophys. Acta. 2007;1768:964–975. - PubMed
-
- Gether U, Kobilka BK. G Protein-coupled receptors II. Mechanism of agonist activation. J. Biol. Chem. 1998;273:17979–17982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

