Tyrosine triad at the interface between the Rieske iron-sulfur protein, cytochrome c1 and cytochrome c2 in the bc1 complex of Rhodobacter capsulatus
- PMID: 22306765
- PMCID: PMC3322269
- DOI: 10.1016/j.bbabio.2012.01.013
Tyrosine triad at the interface between the Rieske iron-sulfur protein, cytochrome c1 and cytochrome c2 in the bc1 complex of Rhodobacter capsulatus
Abstract
A triad of tyrosine residues (Y152-154) in the cytochrome c(1) subunit (C1) of the Rhodobacter capsulatus cytochrome bc(1) complex (BC1) is ideally positioned to interact with cytochrome c(2) (C2). Mutational analysis of these three tyrosines showed that, of the three, Y154 is the most important, since its mutation to alanine resulted in significantly reduced levels, destabilization, and inactivation of BC1. A second-site revertant of this mutant that regained photosynthetic capacity was found to have acquired two further mutations-A181T and A200V. The Y152Q mutation did not change the spectral or electrochemical properties of C1, and showed wild-type enzymatic C2 reduction rates, indicating that this mutation did not introduce major structural changes in C1 nor affect overall activity. Mutations Y153Q and Y153A, on the other hand, clearly affect the redox properties of C1 (e.g. by lowering the midpoint potential as much as 117 mV in Y153Q) and the activity by 90% and 50%, respectively. A more conservative Y153F mutant on the other hand, behaves similarly to wild-type. This underscores the importance of an aromatic residue at position Y153, presumably to maintain close packing with P184, which modeling indicates is likely to stabilize the sixth heme ligand conformation.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

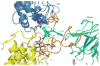








References
-
- Yeates CL, Batchelor JF, Capon EC, Cheesman NJ, Fry M, Hudson AT, Pudney M, Trimming H, Woolven J, Bueno JM, Chicarro J, Fernandez E, Fiandor JM, Gargallo-Viola D, Gomez de las Heras F, Herreros E, Leon ML. Synthesis and Structure–Activity Relationships of 4-Pyridones as Potential Antimalarials. J Med Chem. 2008;51:2845–2852. - PubMed
-
- Barlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. The strobilurin fungicides. Pest Manag Sci. 2002;58:649–662. - PubMed
-
- Jordan DB, Kranis KT, Picollelli MA, Schwartz RS, Sternberg JA, Sun KM. Famoxadone and oxazolidinones: potent inhibitors of cytochrome bc1. Biochem Soc Trans. 1999;27:577–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

