Chimeric calicivirus-like particles elicit specific immune responses in pigs
- PMID: 22306796
- PMCID: PMC7115503
- DOI: 10.1016/j.vaccine.2012.01.069
Chimeric calicivirus-like particles elicit specific immune responses in pigs
Abstract
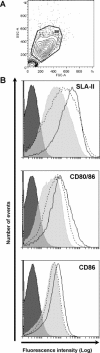
Virus-like particles (VLPs) have received considerable attention due to their potential application in veterinary vaccines and, in particular, VLPs from rabbit haemorrhagic disease virus (RHDV) have successfully shown to be good platforms for inducing immune responses against an inserted foreign epitope in mice. The aim of this study was to assess the immunogenicity of chimeric RHDV-VLPs as vaccine vectors in pigs. For this purpose, we have generated chimeric VLPs containing a well-known T epitope of 3A protein of foot-and-mouth disease virus (FMDV). Firstly, RHDV-VLPs were able to activate immature porcine bone marrow-derived dendritic cells (poBMDCs) in vitro. Secondly, pigs were inoculated twice in a two-week interval with chimeric RHDV-VLPs at different doses intranasally or intramuscularly. One intramuscularly treated group was also inoculated with adjuvant Montanide™ ISA 206 at the same time. Specific IgG and IgA antibodies against RHDV-VLPs were induced and such levels were higher in the adjuvanted group compared with other groups. Interestingly, anti-RHDV-VLP IgA responses were higher in groups inoculated intramuscularly than those that received the VLPs intranasally. Two weeks after the last immunisation, specific IFN-γ-secreting cells against 3A epitope and against RHDV-VLPs were detected in PBMCs by ELISPOT. The adjuvanted group exhibited the highest IFN-γ-secreting cell numbers and lymphoproliferative specific T cell responses against 3A epitope and RHDV-VLP. This is the first immunological report on the potential use of chimeric RHDV-VLPs as antigen carriers in pigs.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Zhao Q., Chen W., Chen Y., Zhang L., Zhang J., Zhang Z. Self-assembled virus-like particles from rotavirus structural protein VP6 for targeted drug delivery. Bioconjug Chem. 2011;22(March (3)):346–352. - PubMed
-
- Roy P., Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4(January–February (1)):5–12. - PubMed
-
- Jennings G.T., Bachmann M.F. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389(May (5)):521–536. - PubMed
-
- Antonis A.F., Bruschke C.J., Rueda P., Maranga L., Casal J.I., Vela C. A novel recombinant virus-like particle vaccine for prevention of porcine parvovirus-induced reproductive failure. Vaccine. 2006;24(June (26)):5481–5490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

