High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis
- PMID: 22306961
- PMCID: PMC3388002
- DOI: 10.1088/1478-3975/9/1/016004
High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis
Abstract
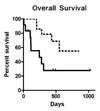
Sampling circulating tumor cells (CTCs) from peripheral blood is ideally accomplished using assays that detect high numbers of cells and preserve them for downstream characterization. We sought to evaluate a method using enrichment free fluorescent labeling of CTCs followed by automated digital microscopy in patients with non-small cell lung cancer. Twenty-eight patients with non-small cell lung cancer and hematogenously seeded metastasis were analyzed with multiple blood draws. We detected CTCs in 68% of analyzed samples and found a propensity for increased CTC detection as the disease progressed in individual patients. CTCs were present at a median concentration of 1.6 CTCs ml⁻¹ of analyzed blood in the patient population. Higher numbers of detected CTCs were associated with an unfavorable prognosis.
Figures








References
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351:781–791. - PubMed
-
- Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, García-Saenz JA, Vidaurreta M, Martíin M, Arroyo M, Sanz-Casla MT, Díaz-Rubio E. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann. of Oncol. 2008;19:935–938. - PubMed
-
- Ghossein RA, Scher HI, Gerald WL, Kelly WK, Curely T, Amsterdam A, Zhang ZF, Rosai J. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J. Clin. Oncol. 1995;13:1195–1200. - PubMed
-
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–1563. - PubMed
-
- Gervasoni A, Sandri MT, Nascimbeni R, Zorzino L, Cassatella MC, Baglioni L, Panigara S, Gervasi M, Di Lorenzo D, Parolini O. Comparison of three distinct methods for the detection of circulating tumor cells in colorectal cancer patients. Oncol. Rep. 2011;25:1669–1703. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
