ApoA1: mimetic peptide reverses adipocyte dysfunction in vivo and in vitro via an increase in heme oxygenase (HO-1) and Wnt10b
- PMID: 22306989
- PMCID: PMC3318105
- DOI: 10.4161/cc.11.4.19125
ApoA1: mimetic peptide reverses adipocyte dysfunction in vivo and in vitro via an increase in heme oxygenase (HO-1) and Wnt10b
Abstract
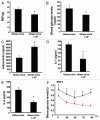
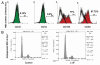
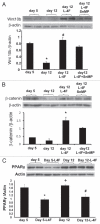
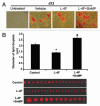
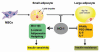
Insulin resistance is a risk factor in the development of type 2 diabetes and is a major cause of atherosclerosis. Reduction in heme oxygenase (HO-1) has been shown to exacerbate vascular dysfunction and insulin resistance in obese mice and involves a decrease in adiponectin levels. Adiponectin is released from mesenchymal stem cell (MSC)-derived adipocytes, its levels are decreased in type 2 diabetes. We hypothesized that the apoA1 mimetic peptide, L-4F, will target the expression of the HO-1-adiponectin axis and reverse adipocyte dysfunction both in vivo and in vitro. The administration of L-4F [2 mg/Kg/daily (i.p.) for 4-week to 8-week-old obese (ob) mice restored adipocyte function, increased adiponectin release (p < 0.05) and decreased the levels of IL-1 and IL-6 (p < 0.05)]. These perturbations were associated with an increase in insulin sensitivity (p < 0.01 vs. untreated ob mice) and decreased glucose levels (309 + 42 vs. 201 + 8 mg/d after L-4F treatment). Treatment of both mesenchymal stem cell (MSC)-derived adipocytes with L-4F (50 μg/ml) increased adiponectin (p < 0.05), decreased IL-1 and IL-6 (p < 0.05) levels and increased MSC-derived adipocyte cell numbers by 50% in S phase (p < 0.05). MSC-derived adipocytes treated with L-4F increased WNT10b and decreased Peg 1/Mest. Inhibition of HO activity reversed the decrease in the adipogenic response gene, Peg 1/Mest. An increase of HO-1 expression by L-4F increased insulin-receptor phosphorylation. These findings support the hypothesis that L-4F increases early adipocyte markers, HO-1-adiponectin, WNT10b and decreases Peg1/Mest, negative regulators of adipocyte differentiation.
Figures
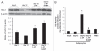
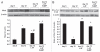
References
-
- Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. 2007;21:149–157. doi: 10.1016/j.jdiacomp.2005.12.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
