BRCA1-directed, enhanced and aberrant homologous recombination: mechanism and potential treatment strategies
- PMID: 22306997
- PMCID: PMC3318103
- DOI: 10.4161/cc.11.4.19212
BRCA1-directed, enhanced and aberrant homologous recombination: mechanism and potential treatment strategies
Abstract
Despite intense studies, questions still remain regarding the molecular mechanisms leading to the development of hereditary breast and ovarian cancers. Research focused on elucidating the role of the breast cancer susceptibility gene 1 (BRCA1) in the DNA damage response may be of the most critical importance to understanding these processes. The BRCA1 protein has an N-terminal RING domain possessing E3 ubiquitinligase activity and a C-terminal BRCT domain involved in binding specific phosphoproteins. These domains are involved directly or indirectly in DNA double-strand break (DSB) repair. As the two terminal domains of BRCA1 represent two separate entities, understanding how these domains communicate and are functionally altered in regards to DSB repair is critical for understanding the development of BRCA1-related breast and ovarian cancers and for developing novel therapeutics. Herein, we review recent findings of how altered functions of these domains might lead to cancer through a mechanism of increased aberrant homologous recombination and possible implications for the development of BRCA1 inhibitors.
Figures
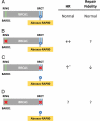

 dynamic interactions between the RING, BRCT, SQ cluster and coiled-coil (CC) domains.
dynamic interactions between the RING, BRCT, SQ cluster and coiled-coil (CC) domains.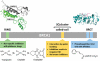
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
