GPCR mediated regulation of synaptic transmission
- PMID: 22307060
- PMCID: PMC3319362
- DOI: 10.1016/j.pneurobio.2012.01.009
GPCR mediated regulation of synaptic transmission
Abstract
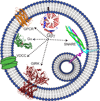
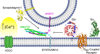
Synaptic transmission is a finely regulated mechanism of neuronal communication. The release of neurotransmitter at the synapse is not only the reflection of membrane depolarization events, but rather, is the summation of interactions between ion channels, G protein coupled receptors, second messengers, and the exocytotic machinery itself which exposes the components within a synaptic vesicle to the synaptic cleft. The focus of this review is to explore the role of G protein signaling as it relates to neurotransmission, as well as to discuss the recently determined inhibitory mechanism of Gβγ dimers acting directly on the exocytotic machinery proteins to inhibit neurotransmitter release.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ahnert-Hilger G, Brautigam M, Gratzl M. Ca2+-stimulated catecholamine release from alpha-toxin-permeabilized PC12 cells: biochemical evidence for exocytosis and its modulation by protein kinase C and G proteins. Biochemistry. 1987;26(24):7842–7848. - PubMed
-
- Albert PR, Robillard L. G protein specificity: traffic direction required. Cell. Signal. 2002;14(5):407–418. - PubMed
-
- Albrecht C, Bloss HG, Jackisch R, Feuerstein TJ. Evaluation of autoreceptor-mediated control of [3H]acetylcholine release in rat and human neocortex. Exp. Brain Res. 1999;128(3):383–389. - PubMed
-
- Albsoul-Younes AM, Sternweis PM, Zhao P, Nakata H, Nakajima S, Nakajima Y, Kozasa T. Interaction sites of the G protein beta subunit with brain G protein-coupled inward rectifier K+ channel. J. Biol. Chem. 2001;276(16):12712–12717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

