Differential biofilm formation and chemical disinfection resistance of sessile cells of Listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions
- PMID: 22307304
- PMCID: PMC3318796
- DOI: 10.1128/AEM.07099-11
Differential biofilm formation and chemical disinfection resistance of sessile cells of Listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica) conditions
Abstract
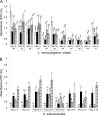
This study aimed to investigate the possible influence of bacterial intra- and interspecies interactions on the ability of Listeria monocytogenes and Salmonella enterica to develop mixed-culture biofilms on an abiotic substratum, as well as on the subsequent resistance of sessile cells to chemical disinfection. Initially, three strains from each species were selected and left to attach and form biofilms on stainless steel (SS) coupons incubated at 15°C for 144 h, in periodically renewable tryptone soy broth (TSB), under either monoculture or mixed-culture (mono-/dual-species) conditions. Following biofilm formation, mixed-culture sessile communities were subjected to 6-min disinfection treatments with (i) benzalkonium chloride (50 ppm), (ii) sodium hypochlorite (10 ppm), (iii) peracetic acid (10 ppm), and (iv) a mixture of hydrogen peroxide (5 ppm) and peracetic acid (5 ppm). Results revealed that both species reached similar biofilm counts (ca. 10(5) CFU cm(-2)) and that, in general, interspecies interactions did not have any significant effect either on the biofilm-forming ability (as this was assessed by agar plating enumeration of the mechanically detached biofilm bacteria) or on the antimicrobial resistance of each individual species. Interestingly, pulsed-field gel electrophoresis (PFGE) analysis clearly showed that the three L. monocytogenes strains did not contribute at the same level either to the formation of mixed-culture sessile communities (mono-/dual species) or to their antimicrobial recalcitrance. Additionally, the simultaneous existence inside the biofilm structure of S. enterica cells seemed to influence the occurrence and resistance pattern of L. monocytogenes strains. In sum, this study highlights the impact of microbial interactions taking place inside a mixed-culture sessile community on both its population dynamics and disinfection resistance.
Figures
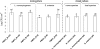
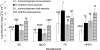


Similar articles
-
Dynamics of biofilm formation by Listeria monocytogenes on stainless steel under mono-species and mixed-culture simulated fish processing conditions and chemical disinfection challenges.Int J Food Microbiol. 2018 Feb 21;267:9-19. doi: 10.1016/j.ijfoodmicro.2017.12.020. Epub 2017 Dec 19. Int J Food Microbiol. 2018. PMID: 29275280
-
Co-culture with Listeria monocytogenes within a dual-species biofilm community strongly increases resistance of Pseudomonas putida to benzalkonium chloride.PLoS One. 2013 Oct 10;8(10):e77276. doi: 10.1371/journal.pone.0077276. eCollection 2013. PLoS One. 2013. PMID: 24130873 Free PMC article.
-
Listeria monocytogenes and Salmonella enterica enteritidis biofilms susceptibility to different disinfectants and stress-response and virulence gene expression of surviving cells.Microb Drug Resist. 2011 Jun;17(2):181-9. doi: 10.1089/mdr.2010.0183. Epub 2011 Mar 9. Microb Drug Resist. 2011. PMID: 21388333
-
The mixed biofilm formed by Listeria monocytogenes and other bacteria: Formation, interaction and control strategies.Crit Rev Food Sci Nutr. 2024;64(24):8570-8586. doi: 10.1080/10408398.2023.2200861. Epub 2023 Apr 17. Crit Rev Food Sci Nutr. 2024. PMID: 37070220 Review.
-
Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens.Front Microbiol. 2015 Aug 20;6:841. doi: 10.3389/fmicb.2015.00841. eCollection 2015. Front Microbiol. 2015. PMID: 26347727 Free PMC article. Review.
Cited by
-
High-level biocidal products effectively eradicate pathogenic γ-proteobacteria biofilms from aquaculture facilities.Aquaculture. 2021 Feb 15;532:736004. doi: 10.1016/j.aquaculture.2020.736004. Aquaculture. 2021. PMID: 39175494 Free PMC article.
-
Pathogens protection against the action of disinfectants in multispecies biofilms.Front Microbiol. 2015 Jul 14;6:705. doi: 10.3389/fmicb.2015.00705. eCollection 2015. Front Microbiol. 2015. PMID: 26236291 Free PMC article. Review.
-
All Treatment Parameters Affect Environmental Surface Sanitation Efficacy, but Their Relative Importance Depends on the Microbial Target.Appl Environ Microbiol. 2020 Dec 17;87(1):e01748-20. doi: 10.1128/AEM.01748-20. Print 2020 Dec 17. Appl Environ Microbiol. 2020. PMID: 33097504 Free PMC article.
-
Biofilm formation by Staphylococcus aureus and Salmonella spp. under mono and dual-species conditions and their sensitivity to cetrimonium bromide, peracetic acid and sodium hypochlorite.Braz J Microbiol. 2018 Apr-Jun;49(2):310-319. doi: 10.1016/j.bjm.2017.08.002. Epub 2017 Oct 13. Braz J Microbiol. 2018. PMID: 29100930 Free PMC article.
-
Biofilm Formation of Listeria monocytogenes and Pseudomonas aeruginosa in a Simulated Chicken Processing Environment.Foods. 2022 Jun 28;11(13):1917. doi: 10.3390/foods11131917. Foods. 2022. PMID: 35804733 Free PMC article.
References
-
- Annous BA, Fratamico PM, Smith JL. 2009. Scientific status summary. J. Food Sci. 74:24–37 - PubMed
-
- Bremer PJ, Monk I, Butler R. 2002. Inactivation of Listeria monocytogenes/Flavobacterium spp. biofilms using chlorine: impact of substrate, pH, time and concentration. Lett. Appl. Microbiol. 35:321–325 - PubMed
-
- Bremer PJ, Monk I, Osborne CM. 2001. Survival of Listeria monocytogenes attached to stainless steel surfaces in the presence or absence of Flavobacterium spp. J. Food Prot. 64:1369–1376 - PubMed
-
- Brooks JD, Flint SH. 2008. Biofilms in the food industry: problems and potential solutions. Int. J. Food Sci. Technol. 43:2163–2176
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

