Pathogenicity and infection cycle of Vibrio owensii in larviculture of the ornate spiny lobster (Panulirus ornatus)
- PMID: 22307306
- PMCID: PMC3318833
- DOI: 10.1128/AEM.07274-11
Pathogenicity and infection cycle of Vibrio owensii in larviculture of the ornate spiny lobster (Panulirus ornatus)
Abstract
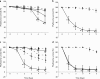
The type strain of Vibrio owensii (DY05) was isolated during an epizootic of aquaculture-reared larvae (phyllosomas) of the ornate spiny lobster (Panulirus ornatus). V. owensii DY05 was formally demonstrated to be the etiological agent of a disease causing rapid and reproducible larval mortality with pathologies similar to those seen during disease epizootics. Vectored challenge via the aquaculture live feed organism Artemia (brine shrimp) caused consistent cumulative mortality rates of 84 to 89% after 72 h, in contrast to variable mortality rates seen after immersion challenge. Histopathological examination of vector-challenged phyllosomas revealed bacterial proliferation in the midgut gland (hepatopancreas) concomitant with epithelial cell necrosis. A fluorescent-protein-labeled V. owensii DY05 transconjugant showed dispersal of single cells in the foregut and hepatopancreas 6 h postexposure, leading to colonization of the entire hepatopancreas within 18 h and eventually systemic infection. V. owensii DY05 is a marine enteropathogen highly virulent to P. ornatus phyllosoma that uses vector-mediated transmission and release from host association to a planktonic existence to perpetuate transfer. This understanding of the infection process will improve targeted biocontrol strategies and enhance the prospects of commercially viable larviculture for this valuable spiny lobster species.
Figures



References
-
- Aguirre-Guzmán A, et al. 2010. Pathogenicity and infection route of Vibrio parahaemolyticus in American white shrimp, Litopenaeus vannamei. J. World Aquacult. Soc. 41:464–470
-
- Aguirre-Guzmán A, Vázquez-Juárez R, Ascencio F. 2001. Differences in the susceptibility of American white shrimp larval substages (Litopenaeus vannamei) to four Vibrio species. J. Invertebr. Pathol. 78:215–219 - PubMed
-
- Austin B, Zhang X-H. 2006. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 43:119–124 - PubMed
-
- Austin B, Austin D, Sutherland R, Thompson F, Swings J. 2005. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ. Microbiol. 7:1488–1495 - PubMed
-
- Bourne DG, et al. 2004. Microbial community dynamics in a larval aquaculture system of the tropical rock lobster, Panulirus ornatus. Aquaculture 242:31–51
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

