Effect of soybean coumestrol on Bradyrhizobium japonicum nodulation ability, biofilm formation, and transcriptional profile
- PMID: 22307307
- PMCID: PMC3318843
- DOI: 10.1128/AEM.07336-11
Effect of soybean coumestrol on Bradyrhizobium japonicum nodulation ability, biofilm formation, and transcriptional profile
Abstract
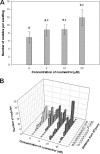
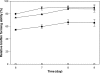
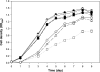
Flavonoids, secondary plant metabolites which mainly have a polyphenolic structure, play an important role in plant-microbe communications for nitrogen-fixing symbiosis. Among 10 polyphenolic compounds isolated from soybean roots in our previous study, coumestrol showed the highest antioxidant activity. In this study, its effect on the soybean nodulation was tested. The soybean symbiont Bradyrhizobium japonicum USDA110 pretreated with 20 μM coumestrol enhanced soybean nodulation by increasing the number of nodules 1.7-fold compared to the control. We also tested the effect of coumestrol on B. japonicum biofilm formation. At a concentration of 2 μM, coumestrol caused a higher degree of biofilm formation than two major soybean isoflavonoids, genistein and daidzein, although no biofilm formation was observed at a concentration of 20 μM each compound. A genome-wide transcriptional analysis was performed to obtain a comprehensive snapshot of the B. japonicum response to coumestrol. When the bacterium was incubated in 20 μM coumestrol for 24 h, a total of 371 genes (139 upregulated and 232 downregulated) were differentially expressed at a 2-fold cutoff with a q value of less than 5%. No common nod gene induction was found in the microarray data. However, quantitative reverse transcription-PCR (qRT-PCR) data showed that incubation for 12 h resulted in a moderate induction (ca. 2-fold) of nodD1 and nodABC, indicating that soybean coumestrol is a weak inducer of common nod genes. In addition, disruption of nfeD (bll4952) affected the soybean nodulation by an approximate 30% reduction in the average number of nodules.
Figures
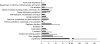
Similar articles
-
Nodulation gene regulation and quorum sensing control density-dependent suppression and restriction of nodulation in the Bradyrhizobium japonicum-soybean symbiosis.Appl Environ Microbiol. 2008 Jun;74(12):3749-56. doi: 10.1128/AEM.02939-07. Epub 2008 Apr 25. Appl Environ Microbiol. 2008. PMID: 18441104 Free PMC article.
-
Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum.Plant J. 2006 Oct;48(2):261-73. doi: 10.1111/j.1365-313X.2006.02874.x. Plant J. 2006. PMID: 17018035
-
Characterization of MocR, a GntR-like transcriptional regulator, in Bradyrhizobium japonicum: its impact on motility, biofilm formation, and soybean nodulation.J Microbiol. 2015 Aug;53(8):518-25. doi: 10.1007/s12275-015-5313-z. Epub 2015 Jul 31. J Microbiol. 2015. PMID: 26224454
-
Effects of indole-3-acetic acid on the transcriptional activities and stress tolerance of Bradyrhizobium japonicum.PLoS One. 2013 Oct 2;8(10):e76559. doi: 10.1371/journal.pone.0076559. eCollection 2013. PLoS One. 2013. PMID: 24098533 Free PMC article.
-
Impact of glyphosate on the Bradyrhizobium japonicum symbiosis with glyphosate-resistant transgenic soybean: a minireview.J Environ Qual. 2004 May-Jun;33(3):825-31. doi: 10.2134/jeq2004.0825. J Environ Qual. 2004. PMID: 15224916 Review.
Cited by
-
Production of the plant hormone gibberellin by rhizobia increases host legume nodule size.ISME J. 2022 Jul;16(7):1809-1817. doi: 10.1038/s41396-022-01236-5. Epub 2022 Apr 12. ISME J. 2022. PMID: 35414717 Free PMC article.
-
Effects of the Bradyrhizobium japonicum waaL (rfaL) Gene on Hydrophobicity, Motility, Stress Tolerance, and Symbiotic Relationship with Soybeans.Int J Mol Sci. 2015 Jul 23;16(8):16778-91. doi: 10.3390/ijms160816778. Int J Mol Sci. 2015. PMID: 26213919 Free PMC article.
-
Can Leaves and Stems of Rubus idaeus L. Handle Candida albicans Biofilms?Pharmaceuticals (Basel). 2020 Dec 18;13(12):477. doi: 10.3390/ph13120477. Pharmaceuticals (Basel). 2020. PMID: 33353173 Free PMC article.
-
Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of pathogenic bacteria.Appl Environ Microbiol. 2015 Jun;81(11):3782-92. doi: 10.1128/AEM.00239-15. Epub 2015 Mar 27. Appl Environ Microbiol. 2015. PMID: 25819960 Free PMC article.
-
Comprehensive RNA sequencing and co-expression network analysis to complete the biosynthetic pathway of coumestrol, a phytoestrogen.Sci Rep. 2019 Feb 13;9(1):1934. doi: 10.1038/s41598-018-38219-6. Sci Rep. 2019. PMID: 30760815 Free PMC article.
References
-
- Banfalvi Z, Nieuwkoop A, Schell M, Besl L, Stacey G. 1988. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol. Gen. Genet. 214:420–424 - PubMed
-
- Bergersen FJ. 1961. The growth of rhizobium in synthetic media. Aust. J. Biol. Sci. 14:349–360
-
- Bickoff EM, et al. 1957. Coumestrol, a new estrogen isolated from forage crops. Science 126:969–970 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

