Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years
- PMID: 22307384
- PMCID: PMC3573681
- DOI: 10.1177/1352458512436594
Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years
Abstract
Background: Teriflunomide, an oral disease-modifying therapy in development for patients with relapsing forms of multiple sclerosis (RMS), was well tolerated and effective in reducing magnetic resonance imaging (MRI) lesions in 179 RMS patients in a phase 2 36-week, placebo-controlled study.
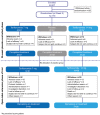
Methods: A total of 147 patients who completed the core study entered an open-label extension. Teriflunomide patients continued their assigned dose, and placebo patients were re-allocated to teriflunomide, 7 mg/day or 14 mg/day. An interim analysis was performed at a cut-off on January 8 2010.
Results: The mean and median duration of study treatment, including both the core and extension phase, from baseline to the interim cut-off, was 5.6 years (standard deviation: 2.7 years) and 7.1 years (range: 0.05-8.5 years), respectively. Of 147 patients, 62 (42.2%) discontinued (19% due to treatment-emergent adverse events (TEAEs)). The most common TEAEs were mild infections, fatigue, sensory disturbances and diarrhoea. No serious opportunistic infections occurred, with no discontinuations due to infection. Asymptomatic alanine aminotransferase increases (≤3× upper limit of normal (ULN)) were common (7 mg, 64.2%; 14 mg, 62.1%); increases >3×ULN were similar across groups (7 mg, 12.3%; 14 mg, 12.1%). Mild decreases in neutrophil counts occurred; none led to discontinuation. The incidence of malignancies was comparable to that of the general population, and cases were not reminiscent of those observed in immunocompromised patients. Annualised relapse rates remained low, minimal disability progression was observed, with a dose-dependent benefit with teriflunomide 14 mg for several MRI parameters.
Conclusion: Teriflunomide had a favourable safety profile for up to 8.5 years.
Trial registration: ClinicalTrials.gov NCT00228163.
Conflict of interest statement
David Li has received research funding from the Canadian Institute of Health Research and Multiple Sclerosis Society of Canada, has consulted for Genzyme and Novartis, and is the Director of the UBC MS/MRI Research Group, which has been contracted to perform central analysis of MRI scans for therapeutic trials for Angiotech, Bayer, Berlex-Schering, BioMS, Boehringer Ingelheim, Centocor, Daiichi Sankyo, Genentech, Hoffmann-LaRoche, Merck Serono, Perceptives, Schering-Plough, Teva Neurosciences, sanofi-aventis and Transition Therapeutics.
Mark Freedman has received grant support from Genzyme and has acted as an advisor/consultant/steering committee member or speaker for Bayer, Biogen Idec, Teva, Merck Serono, Novartis, and sanofi-aventis.
Philippe Truffinet, Hadj Benzerdjeb and Dazhe Wang are employees of sanofi-aventis.
Amit Bar-Or has received personal compensation for consulting, serving on scientific advisory boards and/or speaking activities from: Bayer, Bayhill Therapeutics, Berlex, Biogen Idec, BioMS, Diogenix, Eli-Lilly, Genentech, GSK, Guthy-Jackson/GGF, Merck Serono, Novartis, Ono Pharmacia, Roche, sanofi-aventis, Teva Neurosciences and Wyeth.
Anthony Traboulsee has received personal compensation for membership of data safety monitoring boards (Merck Serono and Daiichi Sankyo), co-chairing a symposium (Merck Serono), chairing a symposium (Teva Neurosciences), speaking (Bayer Health Care), and membership of an editorial advisory board (Neura).
Lucy Reiman is an employee of Fishawack Communications Ltd., contracted to provide editorial services for sanofi-aventis.
Paul O’Connor has received consulting fees and/or research support for MS trials from Actelion, Bayer, Biogen Idec, BioMS, Cognosci, Daiichi Sankyo, EMD Serono, Genentech, Genmab, Novartis, Roche, sanofi-aventis, Teva Neurosciences and Warburg Pincus.
Figures

References
-
- Fox RI, Herrmann ML, Frangou CG, et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol 1999; 93: 198–208 - PubMed
-
- Ruckemann K, Fairbanks LD, Carrey EA, et al. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem 1998; 273: 21682–21691 - PubMed
-
- Petty M, Lee L, Ying X, et al. Teriflunomide treatment reduces infiltration of macrophages, T cells and B cells, and increases survival of oligodendrocytes in the spinal cord of the Dark Agouti rat model of Experimental Allergic Encephalomyelitis. Presented at the Annual Meeting of the American Academy of Neurology, Toronto, Canada, April 10–17 2010
-
- McMonagle-Strucko K, Hanak S, Pu SF, et al. Teriflunomide reduces neurological behaviour and pathology in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. Presented at the 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Dusseldorf, Germany, September 9–12 2009
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

