Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial
- PMID: 22307566
- PMCID: PMC3333836
- DOI: 10.1158/1940-6207.CAPR-11-0251
Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial
Abstract
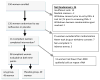
Soy isoflavone consumption may protect against breast cancer development. We conducted a phase IIB trial of soy isoflavone supplementation to examine its effect on breast epithelial proliferation and other biomarkers in the healthy high-risk breast. One hundred and twenty-six consented women underwent a random fine-needle aspiration (rFNA); those with 4,000 or more epithelial cells were randomized to a double-blind 6-month intervention of mixed soy isoflavones (PTIG-2535) or placebo, followed by repeat rFNA. Cells were examined for Ki-67 labeling index and atypia. Expression of 28 genes related to proliferation, apoptosis, and estrogenic effect was measured using quantitative reverse transcriptase PCR. Hormone and protein levels were measured in nipple aspirate fluid (NAF). All statistical tests were two-sided. Ninety-eight women were evaluable for Ki-67 labeling index. In 49 treated women, the median Ki-67 labeling index was 1.18 at entry and 1.12 post intervention, whereas in 49 placebo subjects, it was 0.97 and 0.92 (P for between-group change: 0.32). Menopausal stratification yielded similar results between groups, but within premenopausal soy-treated women, Ki-67 labeling index increased from 1.71 to 2.18 (P = 0.04). We saw no treatment effect on cytologic atypia or NAF parameters. There were significant increases in the expression of 14 of 28 genes within the soy, but not the control group, without significant between-group differences. Plasma genistein values showed excellent compliance. A 6-month intervention of mixed soy isoflavones in healthy, high-risk adult Western women did not reduce breast epithelial proliferation, suggesting a lack of efficacy for breast cancer prevention and a possible adverse effect in premenopausal women.
Trial registration: ClinicalTrials.gov NCT00290758.
©2011 AACR.
Figures

Comment in
-
Soy isoflavones for breast cancer risk reduction.Cancer Prev Res (Phila). 2012 Jul;5(7):984-5; author reply 986-7. doi: 10.1158/1940-6207.CAPR-12-0102. Epub 2012 Jun 12. Cancer Prev Res (Phila). 2012. PMID: 22693167 No abstract available.
Similar articles
-
Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: a phase I clinical trial.Menopause. 2008 Jul-Aug;15(4 Pt 1):684-92. doi: 10.1097/gme.0b013e318167b8f2. Menopause. 2008. PMID: 18446090 Free PMC article. Clinical Trial.
-
Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women.Cancer Epidemiol Biomarkers Prev. 1996 Oct;5(10):785-94. Cancer Epidemiol Biomarkers Prev. 1996. PMID: 8896889
-
Soy isoflavones decrease fibroglandular breast tissue measured by magnetic resonance imaging in premenopausal women: A 2-year randomized double-blind placebo controlled clinical trial.Clin Nutr ESPEN. 2022 Dec;52:158-168. doi: 10.1016/j.clnesp.2022.10.007. Epub 2022 Oct 26. Clin Nutr ESPEN. 2022. PMID: 36513449 Free PMC article. Clinical Trial.
-
Effect of the Intake of Isoflavones on Risk Factors of Breast Cancer-A Systematic Review of Randomized Controlled Intervention Studies.Nutrients. 2021 Jul 5;13(7):2309. doi: 10.3390/nu13072309. Nutrients. 2021. PMID: 34371819 Free PMC article.
-
Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview.Int J Mol Sci. 2021 Mar 22;22(6):3212. doi: 10.3390/ijms22063212. Int J Mol Sci. 2021. PMID: 33809928 Free PMC article. Review.
Cited by
-
Antimetabolic Effects of Polyphenols in Breast Cancer Cells: Focus on Glucose Uptake and Metabolism.Front Nutr. 2018 Apr 16;5:25. doi: 10.3389/fnut.2018.00025. eCollection 2018. Front Nutr. 2018. PMID: 29713632 Free PMC article. Review.
-
Relationship of serum levels and dietary intake of isoflavone, and the novel bacterium Slackia sp. strain NATTS with the risk of prostate cancer: a case-control study among Japanese men.Int Urol Nephrol. 2016 Sep;48(9):1453-60. doi: 10.1007/s11255-016-1335-7. Epub 2016 Jun 4. Int Urol Nephrol. 2016. PMID: 27262851
-
Genistein Improves Skin Flap Viability in Rats: A Preliminary In Vivo and In Vitro Investigation.Molecules. 2018 Jul 4;23(7):1637. doi: 10.3390/molecules23071637. Molecules. 2018. PMID: 29973576 Free PMC article.
-
Drug repurposing for cancer therapy.Signal Transduct Target Ther. 2024 Apr 19;9(1):92. doi: 10.1038/s41392-024-01808-1. Signal Transduct Target Ther. 2024. PMID: 38637540 Free PMC article. Review.
-
May isoflavones prevent breast cancer risk?Rev Assoc Med Bras (1992). 2022 Nov 28;68(11):1487-1489. doi: 10.1590/1806-9282.2EDITR11. eCollection 2022. Rev Assoc Med Bras (1992). 2022. PMID: 36449762 Free PMC article. No abstract available.
References
-
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–62. - PubMed
-
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–41. - PubMed
-
- Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–5. - PubMed
-
- Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100(9):1800–6. - PubMed
-
- Yen TW, Hunt KK, Mirza NQ, Thomas ES, Singletary SE, Babiera GV, et al. Physician recommendations regarding tamoxifen and patient utilization of tamoxifen after surgery for ductal carcinoma in situ. Cancer. 2004;100(5):942–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

