Virus-induced gene silencing (VIGS) in Cysticapnos vesicaria, a zygomorphic-flowered Papaveraceae (Ranunculales, basal eudicots)
- PMID: 22307568
- PMCID: PMC3310490
- DOI: 10.1093/aob/mcs008
Virus-induced gene silencing (VIGS) in Cysticapnos vesicaria, a zygomorphic-flowered Papaveraceae (Ranunculales, basal eudicots)
Abstract
Background and aims: Studies of evolutionary diversification in the basal eudicot family Papaveraceae, such as the transition from actinomorphy to zygomorphy, are hampered by the lack of comparative functional studies. So far, gene silencing methods are only available in the actinomorphic species Eschscholzia californica and Papaver somniferum. This study addresses the amenability of Cysticapnos vesicaria, a derived fumitory with zygomorphic flowers, to virus-induced gene silencing (VIGS), and describes vegetative and reproductive traits in this species.
Methods: VIGS-mediated downregulation of the C. vesicaria PHYTOENE DESATURASE gene (CvPDS) and of the FLORICAULA gene CvFLO was carried out using Agrobacterium tumefaciens transfer of Tobacco rattle virus (TRV)-based vectors. Wild-type and vector-treated plants were characterized using reverse transcription-PCR (RT-PCR), in situ hybridization, and macroscopic and scanning electron microscopic imaging.
Key results: Cysticapnos vesicaria germinates rapidly, can be grown at high density, has a short life cycle and is self-compatible. Inoculation of C. vesicaria with a CvPDS-VIGS vector resulted in strong photobleaching of green parts and reduction of endogenous CvPDS transcript levels. Gene silencing persisted during inflorescence development until fruit set. Inoculation of plants with CvFLO-VIGS affected floral phyllotaxis, symmetry and floral organ identities.
Conclusions: The high penetrance, severity and stability of pTRV-mediated silencing, including the induction of meristem-related phenotypes, make C. vesicaria a very promising new focus species for evolutionary-developmental (evo-devo) studies in the Papaveraceae. This now enables comparative studies of flower symmetry, inflorescence determinacy and other traits that diversified in the Papaveraceae.
Figures


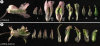

References
-
- Allen KD, Sussex IM. Falsiflora and anantha control early stages of floral meristem development in tomato (Lycopersicon esculentum Mill.) Planta. 1996;200:254–264.
-
- APG III. An update of the Angiosperms Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121.
-
- Ballerini ES, Kramer EM. Environmental and molecular analysis of the floral transition in the lower eudicot Aquilegia formosa. EvoDevo. 2011;2(4) http://dx.doi.org/10.1186/2041-9139-2-4 . - PMC - PubMed
-
- Bartholmes C. Regulation of morphogenesis of lateral organs in the basal eudicot. 2011 Eschscholzia californica. PhD dissertation, Ohio University, USA.
-
- Baulcombe DC. Fast forward genetics based on virus-induced gene silencing. Current Opinion in Plant Biology. 1999;2:109–113. - PubMed

