Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial
- PMID: 22307571
- PMCID: PMC3743415
- DOI: 10.1001/jama.2012.137
Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial
Abstract
Context: The amount of enteral nutrition patients with acute lung injury need is unknown.
Objective: To determine if initial lower-volume trophic enteral feeding would increase ventilator-free days and decrease gastrointestinal intolerances compared with initial full enteral feeding.
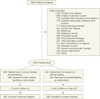
Design, setting, and participants: The EDEN study, a randomized, open-label, multicenter trial conducted from January 2, 2008, through April 12, 2011. Participants were 1000 adults within 48 hours of developing acute lung injury requiring mechanical ventilation whose physicians intended to start enteral nutrition at 44 hospitals in the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network.
Interventions: Participants were randomized to receive either trophic or full enteral feeding for the first 6 days. After day 6, the care of all patients who were still receiving mechanical ventilation was managed according to the full feeding protocol.
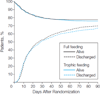
Main outcome measures: Ventilator-free days to study day 28.
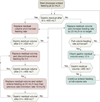
Results: Baseline characteristics were similar between the trophic-feeding (n = 508) and full-feeding (n = 492) groups. The full-feeding group received more enteral calories for the first 6 days, about 1300 kcal/d compared with 400 kcal/d (P < .001). Initial trophic feeding did not increase the number of ventilator-free days (14.9 [95% CI, 13.9 to 15.8] vs 15.0 [95% CI, 14.1 to 15.9]; difference, -0.1 [95% CI, -1.4 to 1.2]; P = .89) or reduce 60-day mortality (23.2% [95% CI, 19.6% to 26.9%] vs 22.2% [95% CI, 18.5% to 25.8%]; difference, 1.0% [95% CI, -4.1% to 6.3%]; P = .77) compared with full feeding. There were no differences in infectious complications between the groups. Despite receiving more prokinetic agents, the full-feeding group experienced more vomiting (2.2% vs 1.7% of patient feeding days; P = .05), elevated gastric residual volumes (4.9% vs 2.2% of feeding days; P < .001), and constipation (3.1% vs 2.1% of feeding days; P = .003). Mean plasma glucose values and average hourly insulin administration were both higher in the full-feeding group over the first 6 days.
Conclusion: In patients with acute lung injury, compared with full enteral feeding, a strategy of initial trophic enteral feeding for up to 6 days did not improve ventilator-free days, 60-day mortality, or infectious complications but was associated with less gastrointestinal intolerance.
Trial registration: clinicaltrials.gov Identifiers: NCT00609180 and NCT00883948.
Conflict of interest statement
Figures

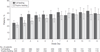


Comment in
-
Nutrition for critically ill patients: how much is enough?JAMA. 2012 Feb 22;307(8):845-6. doi: 10.1001/jama.2012.168. Epub 2012 Feb 5. JAMA. 2012. PMID: 22307570 No abstract available.
-
Feeding, simvastatin, and linezolid.Am J Respir Crit Care Med. 2012 Jul 15;186(2):195-6. doi: 10.1164/rccm.201203-0549RR. Am J Respir Crit Care Med. 2012. PMID: 22798415 Free PMC article. No abstract available.
-
JPEN Journal Club 14. Relative vs Absolute Efficacy.JPEN J Parenter Enteral Nutr. 2015 Aug;39(6):743-5. doi: 10.1177/0148607115583537. JPEN J Parenter Enteral Nutr. 2015. PMID: 26201821 No abstract available.
References
-
- McClave SA, Martindale RG, Vanek VW, et al. A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33(3):277–316. - PubMed
-
- Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian Critical Care Clinical Practice Guidelines Committee. Canadian Clinical Practice Guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27(5):355–373. - PubMed
-
- Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in Canadian ICUs—opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27(1):74–83. - PubMed
-
- Heyland DK, Dhaliwal R, Day A, Jain M, Drover J. Validation of the Canadian Clinical Practice Guidelines for nutrition support in mechanically ventilated, critically ill adult patients: results of a prospective observational study. Crit Care Med. 2004;32(11):2260–2266. - PubMed
-
- Rice TW, Swope T, Bozeman S, Wheeler AP. Variation in enteral nutrition delivery in mechanically ventilated patients. Nutrition. 2005;21(7–8):786–792. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- N01 HR056168/HL/NHLBI NIH HHS/United States
- N01 HR056174/HL/NHLBI NIH HHS/United States
- N01 HR056169/HL/NHLBI NIH HHS/United States
- N01 HR056179/HL/NHLBI NIH HHS/United States
- N01 HR056165/HL/NHLBI NIH HHS/United States
- N01 HR056175/HL/NHLBI NIH HHS/United States
- N01 HR056176/HL/NHLBI NIH HHS/United States
- N01 HR056166/HL/NHLBI NIH HHS/United States
- HHSN268200536179C/PHS HHS/United States
- N01 HR056173/HL/NHLBI NIH HHS/United States
- N01 HR056170/HR/NHLBI NIH HHS/United States
- N01 HR056167/HL/NHLBI NIH HHS/United States
- HHSN268200536165C/PHS HHS/United States
- N01 HR056172/HL/NHLBI NIH HHS/United States
- N01 HR056171/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

