Homeobox gene distal-less is required for neuronal differentiation and neurite outgrowth in the Drosophila olfactory system
- PMID: 22307614
- PMCID: PMC3277119
- DOI: 10.1073/pnas.1016741109
Homeobox gene distal-less is required for neuronal differentiation and neurite outgrowth in the Drosophila olfactory system
Abstract
Vertebrate Dlx genes have been implicated in the differentiation of multiple neuronal subtypes, including cortical GABAergic interneurons, and mutations in Dlx genes have been linked to clinical conditions such as epilepsy and autism. Here we show that the single Drosophila Dlx homolog, distal-less, is required both to specify chemosensory neurons and to regulate the morphologies of their axons and dendrites. We establish that distal-less is necessary for development of the mushroom body, a brain region that processes olfactory information. These are important examples of distal-less function in an invertebrate nervous system and demonstrate that the Drosophila larval olfactory system is a powerful model in which to understand distal-less functions during neurogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
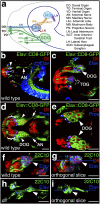
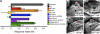
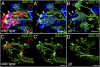
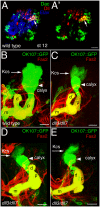
References
-
- Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–4386. - PubMed
-
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. - PubMed
-
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: A review. Chem Senses. 2007;32(1):65–89. - PubMed
-
- Singh R, Singh K. Fine structure of the sensory organs of Drosophila melanogaster Meigen larva (Diptera: Drosophilidae) Int J Insect Morphol Embryol. 1984;13(4):255–273.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

