Scaffold protein Disc large homolog 1 is required for T-cell receptor-induced activation of regulatory T-cell function
- PMID: 22307621
- PMCID: PMC3277153
- DOI: 10.1073/pnas.1110120109
Scaffold protein Disc large homolog 1 is required for T-cell receptor-induced activation of regulatory T-cell function
Abstract
Foxp3(+)CD4(+)CD25(high) regulatory T cell (Treg) suppression of inflammation depends on T-cell receptor-mediated Nuclear Factor of Activated T cells c1 (NFATc1) activation with reduced Akt activity. We investigated the role of the scaffold protein Disc large homolog 1 (Dlgh1) in linking the T-cell receptor to this unique signaling outcome. The Treg immunological synapse (IS) recruited fourfold more Dlgh1 than conventional CD4(+) T-cell IS. Tregs isolated from patients with active rheumatoid arthritis, or treated with tumor necrosis factor-α, displayed reduced function and diminished Dlgh1 recruitment to the IS. Furthermore, Dlgh1 silencing abrogated Treg function, impaired NFATc1 activation, reduced phosphatase and tensin homolog levels, and increased Akt activation. Dlgh1 operates independently of the negative feedback pathway mediated by the related adapter protein Carma1 and thus presents an array of unique targets to selectively manipulate Treg function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
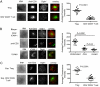

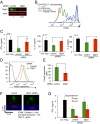
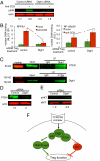
Similar articles
-
Human in vitro-induced regulatory T cells display Dlgh1dependent and PKC-θ restrained suppressive activity.Sci Rep. 2017 Jun 26;7(1):4258. doi: 10.1038/s41598-017-04053-5. Sci Rep. 2017. PMID: 28652577 Free PMC article.
-
DLGH1 is a negative regulator of T-lymphocyte proliferation.Mol Cell Biol. 2007 Nov;27(21):7574-81. doi: 10.1128/MCB.00439-07. Epub 2007 Aug 27. Mol Cell Biol. 2007. PMID: 17724087 Free PMC article.
-
Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells.J Exp Med. 2005 Feb 7;201(3):419-30. doi: 10.1084/jem.20041428. J Exp Med. 2005. PMID: 15699074 Free PMC article.
-
Expression of tumor necrosis factor-α induced protein 8 like-2 contributes to the immunosuppressive property of CD4(+)CD25(+) regulatory T cells in mice.Mol Immunol. 2011 Oct;49(1-2):219-26. doi: 10.1016/j.molimm.2011.08.016. Epub 2011 Oct 2. Mol Immunol. 2011. PMID: 21963221
-
Decreased suppression of CD8+ and CD4+ T cells by peripheral regulatory T cells in generalized vitiligo due to reduced NFATC1 and FOXP3 proteins.Exp Dermatol. 2020 Aug;29(8):759-775. doi: 10.1111/exd.14157. Epub 2020 Aug 11. Exp Dermatol. 2020. PMID: 32682346
Cited by
-
Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability.Nat Immunol. 2015 Feb;16(2):188-96. doi: 10.1038/ni.3077. Epub 2015 Jan 5. Nat Immunol. 2015. PMID: 25559257 Free PMC article.
-
Polarized cells, polarized views: asymmetric cell division in hematopoietic cells.Front Immunol. 2014 Feb 3;5:26. doi: 10.3389/fimmu.2014.00026. eCollection 2014. Front Immunol. 2014. PMID: 24550912 Free PMC article. Review.
-
Treg cells in rheumatoid arthritis: an update.Curr Rheumatol Rep. 2013 Sep;15(9):352. doi: 10.1007/s11926-013-0352-0. Curr Rheumatol Rep. 2013. PMID: 23888361 Review.
-
Polarity gene discs large homolog 1 regulates the generation of memory T cells.Eur J Immunol. 2013 May;43(5):1185-94. doi: 10.1002/eji.201142362. Epub 2013 Apr 9. Eur J Immunol. 2013. PMID: 23436244 Free PMC article.
-
Characterization of in vivo Dlg1 deletion on T cell development and function.PLoS One. 2012;7(9):e45276. doi: 10.1371/journal.pone.0045276. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028902 Free PMC article.
References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2 AR058986/AR/NIAMS NIH HHS/United States
- R56 AI088553/AI/NIAID NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- F32 CA067493/CA/NCI NIH HHS/United States
- R37AI43542/AI/NIAID NIH HHS/United States
- 2P01CA067493/CA/NCI NIH HHS/United States
- R56AI88553/AI/NIAID NIH HHS/United States
- 2RC2AR058986/AR/NIAMS NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- PN2EY01696/EY/NEI NIH HHS/United States
- R37 AI043542/AI/NIAID NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- R01 AI041647/AI/NIAID NIH HHS/United States
- R01AI41647/AI/NIAID NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

