Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS)
- PMID: 22307627
- PMCID: PMC3277168
- DOI: 10.1073/pnas.1113166109
Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS)
Abstract
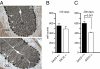
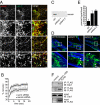
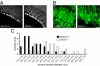
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is a childhood-onset neurological disease resulting from mutations in the SACS gene encoding sacsin, a 4,579-aa protein of unknown function. Originally identified as a founder disease in Québec, ARSACS is now recognized worldwide. Prominent features include pyramidal spasticity and cerebellar ataxia, but the underlying pathology and pathophysiological mechanisms are unknown. We have generated an animal model for ARSACS, sacsin knockout mice, that display age-dependent neurodegeneration of cerebellar Purkinje cells. To explore the pathophysiological basis for this observation, we examined the cell biological properties of sacsin. We show that sacsin localizes to mitochondria in non-neuronal cells and primary neurons and that it interacts with dynamin-related protein 1, which participates in mitochondrial fission. Fibroblasts from ARSACS patients show a hyperfused mitochondrial network, consistent with defects in mitochondrial fission. Sacsin knockdown leads to an overly interconnected and functionally impaired mitochondrial network, and mitochondria accumulate in the soma and proximal dendrites of sacsin knockdown neurons. Disruption of mitochondrial transport into dendrites has been shown to lead to abnormal dendritic morphology, and we observe striking alterations in the organization of dendritic fields in the cerebellum of knockout mice that precedes Purkinje cell death. Our data identifies mitochondrial dysfunction/mislocalization as the likely cellular basis for ARSACS and indicates a role for sacsin in regulation of mitochondrial dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
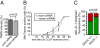

References
-
- Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can J Neurol Sci. 1978;5:61–69. - PubMed
-
- Bouchard JP. Recessive spastic ataxia of Charlevoix-Saguenay. In: de Jong JMBV, editor. Hereditary Neuropathies and Spinocerebellar Atrophies Handbook of Clinical Neurology. Amsterdam: Elsevier; 1991. pp. 451–459.
-
- Dupré N, Bouchard JP, Brais B, Rouleau GA. Hereditary ataxia, spastic paraparesis and neuropathy in the French-Canadian population. Can J Neurol Sci. 2006;33:149–157. - PubMed
-
- Bouhlal Y, Amouri R, El Euch-Fayeche G, Hentati F. Autosomal recessive spastic ataxia of Charlevoix-Saguenay: An overview. Parkinsonism Relat Disord. 2011;17:418–422. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

