IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G
- PMID: 22307629
- PMCID: PMC3277172
- DOI: 10.1073/pnas.1115884109
IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G
Abstract
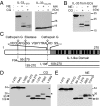
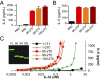
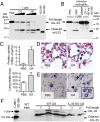
Interleukin-33 (IL-33) (NF-HEV) is a chromatin-associated nuclear cytokine from the IL-1 family, which has been linked to important diseases, including asthma, rheumatoid arthritis, ulcerative colitis, and cardiovascular diseases. IL-33 signals through the ST2 receptor and drives cytokine production in type 2 innate lymphoid cells (ILCs) (natural helper cells, nuocytes), T-helper (Th)2 lymphocytes, mast cells, basophils, eosinophils, invariant natural killer T (iNKT), and natural killer (NK) cells. We and others recently reported that, unlike IL-1β and IL-18, full-length IL-33 is biologically active independently of caspase-1 cleavage and that processing by caspases results in IL-33 inactivation. We suggested that IL-33, which is released upon cellular damage, may function as an endogenous danger signal or alarmin, similar to IL-1α or high-mobility group box 1 protein (HMGB1). Here, we investigated the possibility that IL-33 activity may be regulated by proteases released during inflammation. Using a combination of in vitro and in vivo approaches, we demonstrate that neutrophil serine proteases cathepsin G and elastase can cleave full-length human IL-33(1-270) and generate mature forms IL-33(95-270), IL-33(99-270), and IL-33(109-270). These forms are produced by activated human neutrophils ex vivo, are biologically active in vivo, and have a ~10-fold higher activity than full-length IL-33 in cellular assays. Murine IL-33 is also cleaved by neutrophil cathepsin G and elastase, and both full-length and cleaved endogenous IL-33 could be detected in the bronchoalveolar lavage fluid in an in vivo model of acute lung injury associated with neutrophil infiltration. We propose that the inflammatory microenvironment may exacerbate disease-associated functions of IL-33 through the generation of highly active mature forms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. - PubMed
-
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. - PubMed
-
- Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

