Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle
- PMID: 22307630
- PMCID: PMC3277125
- DOI: 10.1073/pnas.1117885109
Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle
Abstract
Ischemia of the myocardium and lower limbs is a common consequence of arterial disease and a major source of morbidity and mortality in modernized countries. Inducing neovascularization for the treatment of ischemia is an appealing therapeutic strategy for patients for whom traditional treatment modalities cannot be performed or are ineffective. In the past, the stimulation of blood vessel growth was pursued using direct delivery of growth factors, angiogenic gene therapy, or cellular therapy. Although therapeutic angiogenesis holds great promise for treating patients with ischemia, current methods have not found success in clinical trials. Fibroblast growth factor-2 (FGF-2) was one of the first growth factors to be tested for use in therapeutic angiogenesis. Here, we present a method for improving the biological activity of FGF-2 by codelivering the growth factor with a liposomally embedded coreceptor, syndecan-4. This technique was shown to increase FGF-2 cellular signaling, uptake, and nuclear localization in comparison with FGF-2 alone. Delivery of syndecan-4 proteoliposomes also increased endothelial proliferation, migration, and angiogenic tube formation in response to FGF-2. Using an animal model of limb ischemia, syndecan-4 proteoliposomes markedly improved the neovascularization following femoral artery ligation and recovery of perfusion of the ischemic limb. Taken together, these results support liposomal delivery of syndecan-4 as an effective means to improving the potential of using growth factors to achieve therapeutic neovascularization of ischemic tissue.
Conflict of interest statement
Conflict of interest statement: The authors declare a conflict of interest. A.B.B. and E.R.E. have filed a patent on the technology discussed in this paper.
Figures
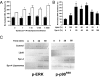

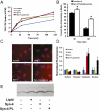
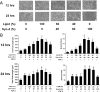
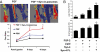
References
-
- Tirziu D, Simons M. Angiogenesis in the human heart: Gene and cell therapy. Angiogenesis. 2005;8:241–251. - PubMed
-
- Simons M, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. - PubMed
-
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. - PubMed
-
- Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. - PubMed
-
- Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

