In-feed antibiotic effects on the swine intestinal microbiome
- PMID: 22307632
- PMCID: PMC3277147
- DOI: 10.1073/pnas.1120238109
In-feed antibiotic effects on the swine intestinal microbiome
Abstract
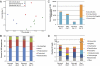
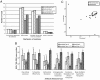
Antibiotics have been administered to agricultural animals for disease treatment, disease prevention, and growth promotion for over 50 y. The impact of such antibiotic use on the treatment of human diseases is hotly debated. We raised pigs in a highly controlled environment, with one portion of the littermates receiving a diet containing performance-enhancing antibiotics [chlortetracycline, sulfamethazine, and penicillin (known as ASP250)] and the other portion receiving the same diet but without the antibiotics. We used phylogenetic, metagenomic, and quantitative PCR-based approaches to address the impact of antibiotics on the swine gut microbiota. Bacterial phylotypes shifted after 14 d of antibiotic treatment, with the medicated pigs showing an increase in Proteobacteria (1-11%) compared with nonmedicated pigs at the same time point. This shift was driven by an increase in Escherichia coli populations. Analysis of the metagenomes showed that microbial functional genes relating to energy production and conversion were increased in the antibiotic-fed pigs. The results also indicate that antibiotic resistance genes increased in abundance and diversity in the medicated swine microbiome despite a high background of resistance genes in nonmedicated swine. Some enriched genes, such as aminoglycoside O-phosphotransferases, confer resistance to antibiotics that were not administered in this study, demonstrating the potential for indirect selection of resistance to classes of antibiotics not fed. The collateral effects of feeding subtherapeutic doses of antibiotics to agricultural animals are apparent and must be considered in cost-benefit analyses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13:7–27. - PubMed
-
- Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: History and mode of action. Poult Sci. 2005;84:634–643. - PubMed
-
- Levy SB. Emergence of antibiotic-resistant bacteria in the intestinal flora of farm inhabitants. J Infect Dis. 1978;137:689–690. - PubMed
-
- Aarestrup FM, Wegener HC. The effects of antibiotic usage in food animals on the development of antimicrobial resistance of importance for humans in Campylobacter and Escherichia coli. Microbes Infect. 1999;1:639–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

