Vena cava and aortic smooth muscle cells express transglutaminases 1 and 4 in addition to transglutaminase 2
- PMID: 22307675
- PMCID: PMC3330795
- DOI: 10.1152/ajpheart.00918.2011
Vena cava and aortic smooth muscle cells express transglutaminases 1 and 4 in addition to transglutaminase 2
Abstract
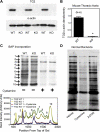
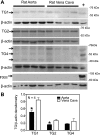
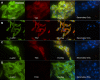
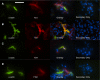
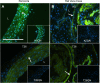
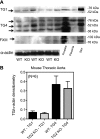
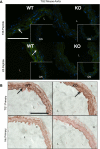
Transglutaminase (TG) function facilitates several vascular processes and diseases. Although many of these TG-dependent vascular processes have been ascribed to the function of TG2, TG2 knockout mice have a mild vascular phenotype. We hypothesized that TGs besides TG2 exist and function in the vasculature. Biotin-pentylamide incorporation, a measure of general TG activity, was similar in wild-type and TG2 knockout mouse aortae, and the general TG inhibitor cystamine reduced biotin-pentylamine incorporation to a greater extent than the TG2-specific inhibitor Z-DON, indicating the presence of other functional TGs. Additionally, 5-hydroxytryptamine-induced aortic contraction, a TG-activity-dependent process, was decreased to a greater extent by general TG inhibitors vs. Z-DON (maximum contraction: cystamine = abolished, monodansylcadaverine = 28.6 ± 14.9%, and Z-DON = 60.2 ± 15.2% vehicle), providing evidence for the importance of TG2-independent activity in the vasculature. TG1, TG2, TG4, and Factor XIII (FXIII) mRNA in rat aortae and vena cavae was detected by RT-PCR. Western analysis detected TG1 and TG4, but not FXIII, in rat aortae and vena cavae and in TG2 knockout and wild-type mouse aortae. Immunostaining confirmed the presence of TG1, TG2, and TG4 in rat aortae and vena cavae, notably in smooth muscle cells; FXIII was absent. K5 and T26, FITC-labeled peptide substrates specific for active TG1 and TG2, respectively, were incorporated into rat aortae and vena cavae and wild-type, but not TG2 knockout, mouse aortae. These studies demonstrate that TG2-independent TG activity exists in the vasculature and that TG1 and TG4 are expressed in vascular tissues.
Figures
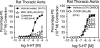

References
-
- Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging 27: 871–879, 2006. - PubMed
-
- Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 96: 119–126, 2005. - PubMed
-
- Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res 99: 86–92, 2006. - PubMed
-
- Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res 45: 271–278, 2008. - PubMed
-
- Baumgartner W, Golenhofen N, Weth A, Hiiragi T, Saint R, Griffin M, Drenckhahn D. Role of transglutaminase 1 in stabilisation of intercellular junctions of the vascular endothelium. Histochem Cell Biol 122: 17–25, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

