Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial
- PMID: 22307942
- PMCID: PMC3396459
- DOI: 10.1136/annrheumdis-2011-200706
Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial
Abstract
Objective: To determine the efficacy and safety of ocrelizumab (OCR) with methotrexate (MTX) in MTX-naive rheumatoid arthritis (RA) patients.
Methods: In a randomised, double-blind, controlled trial, patients received placebo+MTX (MTX; n=210), OCR 200 mg×2+MTX (OCR 200; n=200) or OCR 500 mg×2+MTX (OCR 500; n=203). OCR/placebo (two intravenous infusions) was given on days 1 and 15, with fixed re-treatment scheduled at weeks 24/26, 52/54 and 76/78. Due to early termination of OCR dosing, there was no formal primary end point analysis (change from baseline in modified total Sharp score (ΔmTSS) at week 104). Analyses are reported for week 52 outcomes.
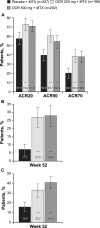
Results: At week 52, treatment with OCR+MTX compared with MTX alone reduced progression of joint damage (mean (SD) change in ΔmTSS: OCR 200, 0.66 (4.51); OCR 500, 0.27 (2.91); MTX alone, 1.59 (4.82); p=0.001 and p=0.003, respectively vs MTX alone) and improved clinical signs and symptoms (American College of Rheumatology 20 response: OCR 200, 73.0%; OCR 500, 71.0%; MTX alone, 57.5%; p<0.005 for each OCR vs MTX alone). Serious infection rates per 100 patient-years were similar with OCR 200 and MTX alone (2.6 (95% CI 0.9 to 6.1) and 3.0 (1.1 to 6.5), respectively), but higher with OCR 500 (7.1 (3.9 to 11.9)).
Conclusions: OCR 200 mg and 500 mg with MTX in MTX-naive patients with RA were effective in inhibiting joint damage progression and improving RA signs and symptoms. OCR 500 mg with MTX was associated with an increased rate of serious infections.
Conflict of interest statement
Figures

References
-
- St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43 - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37 - PubMed
-
- Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82 - PubMed
-
- Edwards JC, Szczepański L, Szechiński J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81 - PubMed
-
- Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006;54:1390–400 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

