Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification
- PMID: 22307978
- PMCID: PMC3361573
- DOI: 10.1002/jbmr.1562
Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification
Abstract
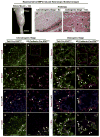
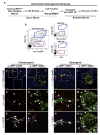
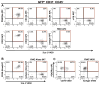
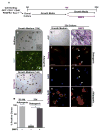
Heterotopic ossification is a debilitating condition that can result from traumatic injury, surgery, or genetic disease. We investigated the cellular origins of heterotopic skeletogenesis in the mouse using lineage tracing and bioassays of heterotopic ossification based on intramuscular transplantation. We identified, characterized, and purified a tissue-resident stem/progenitor cell population that exhibits robust osteogenic potential and represents a major cell-of-origin for heterotopic ossification. These progenitors reside in the interstitium of skeletal muscle and other tissues, and are distinct from the endothelium, which does not exhibit osteogenic activity in response to bone morphogenetic protein 2 (BMP2) stimulation. Intramuscular transplantation, together with clonal analysis in culture, revealed that these progenitors are multipotent, exhibiting the capacity for both BMP-dependent skeletogenic differentiation and spontaneous adipogenic differentiation. Identifying the cells-of-origin responsible for heterotopic ossification provides a potential therapeutic target to treat, mitigate, or prevent this disabling condition.
Copyright © 2012 American Society for Bone and Mineral Research.
Figures


References
-
- Cushner FD, Morwessel RM. Myositis ossificans traumatica. Orthop Rev. 1992;21 (11):1319–26. - PubMed
-
- Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89(3):476–86. - PubMed
-
- Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14(10 Suppl):S191–7. - PubMed
-
- Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37(3):129–36. - PubMed

