Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation
- PMID: 22308018
- PMCID: PMC3770147
- DOI: 10.1002/jbmr.1567
Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation
Abstract
Osteoclasts are thought to be solely responsible for the removal of bone matrix. However, we show here that osteocytes can also remove bone matrix by reversibly remodeling their perilacunar/canalicular matrix during the reproductive cycle. In contrast, no osteocytic remodeling was observed with experimental unloading despite similar degrees of bone loss. Gene array analysis of osteocytes from lactating animals revealed an elevation of genes known to be utilized by osteoclasts to remove bone, including tartrate-resistant acid phosphatase (TRAP) and cathepsin K, that returned to virgin levels upon weaning. Infusion of parathyroid hormone-related peptide (PTHrP), known to be elevated during lactation, induced TRAP activity and cathepsin K expression in osteocytes concurrent with osteocytic remodeling. Conversely, animals lacking the parathyroid hormone type 1 receptor (PTHR1) in osteocytes failed to express TRAP or cathepsin K or to remodel their osteocyte perilacunar matrix during lactation. These studies show that osteocytes remove mineralized matrix through molecular mechanisms similar to those utilized by osteoclasts.
Copyright © 2012 American Society for Bone and Mineral Research.
Conflict of interest statement
The authors have no conflict of interest.
Figures

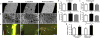

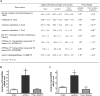

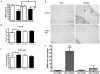

References
-
- Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb J. 2005;19(13):1842–1844. - PubMed
-
- Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–475. - PubMed
-
- Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. Journal of bone and mineral metabolism. 2004;22(6):524–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

