Therapeutic effects of systemic administration of chaperone αB-crystallin associated with binding proinflammatory plasma proteins
- PMID: 22308023
- PMCID: PMC3322989
- DOI: 10.1074/jbc.M111.337691
Therapeutic effects of systemic administration of chaperone αB-crystallin associated with binding proinflammatory plasma proteins
Abstract
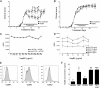
The therapeutic benefit of the small heat shock protein αB-crystallin (HspB5) in animal models of multiple sclerosis and ischemia is proposed to arise from its increased capacity to bind proinflammatory proteins at the elevated temperatures within inflammatory foci. By mass spectral analysis, a common set of ∼70 ligands was precipitated by HspB5 from plasma from patients with multiple sclerosis, rheumatoid arthritis, and amyloidosis and mice with experimental allergic encephalomyelitis. These proteins were distinguished from other precipitated molecules because they were enriched in the precipitate as compared with their plasma concentrations, and they exhibited temperature-dependent binding. More than half of these ligands were acute phase proteins or members of the complement or coagulation cascades. Consistent with this proposal, plasma levels of HspB5 were increased in patients with multiple sclerosis as compared with normal individuals. The combination of the thermal sensitivity of the HspB5 combined with the high local concentration of these ligands at the site of inflammation is proposed to explain the paradox of how a protein believed to exhibit nonspecific binding can bind with some relative apparent selectivity to proinflammatory proteins and thereby modulate inflammation.
Figures


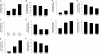

Similar articles
-
Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis.J Biol Chem. 2012 Oct 19;287(43):36423-34. doi: 10.1074/jbc.M112.371229. Epub 2012 Sep 6. J Biol Chem. 2012. PMID: 22955287 Free PMC article.
-
Chaperone activity of α B-crystallin is responsible for its incorrect assignment as an autoantigen in multiple sclerosis.J Immunol. 2011 Apr 1;186(7):4263-8. doi: 10.4049/jimmunol.1003934. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357544 Free PMC article.
-
The role of small heat-shock protein αB-crystalline (HspB5) in COPD pathogenesis.Int J Chron Obstruct Pulmon Dis. 2012;7:633-40. doi: 10.2147/COPD.S34929. Epub 2012 Oct 4. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 23055712 Free PMC article.
-
Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets.FEBS Lett. 2007 Jul 31;581(19):3665-74. doi: 10.1016/j.febslet.2007.04.033. Epub 2007 Apr 24. FEBS Lett. 2007. PMID: 17467701 Review.
-
Cell biological roles of αB-crystallin.Prog Biophys Mol Biol. 2014 Jul;115(1):3-10. doi: 10.1016/j.pbiomolbio.2014.02.005. Epub 2014 Feb 25. Prog Biophys Mol Biol. 2014. PMID: 24576798 Review.
Cited by
-
Crystallins and neuroinflammation: The glial side of the story.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):278-86. doi: 10.1016/j.bbagen.2015.05.023. Epub 2015 Jun 3. Biochim Biophys Acta. 2016. PMID: 26049079 Free PMC article.
-
Alpha beta-crystallin expression and presentation following infection with murine gammaherpesvirus 68.Autoimmunity. 2013 Sep;46(6):399-408. doi: 10.3109/08916934.2013.785535. Epub 2013 Apr 16. Autoimmunity. 2013. PMID: 23586607 Free PMC article.
-
Molecular mechanism of the chaperone function of mini-α-crystallin, a 19-residue peptide of human α-crystallin.Biochemistry. 2015 Jan 20;54(2):505-15. doi: 10.1021/bi5014479. Epub 2014 Dec 26. Biochemistry. 2015. PMID: 25478825 Free PMC article.
-
Alpha B-Crystallin in Muscle Disease Prevention: The Role of Physical Activity.Molecules. 2022 Feb 8;27(3):1147. doi: 10.3390/molecules27031147. Molecules. 2022. PMID: 35164412 Free PMC article. Review.
-
Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human αB-crystallin.Biochemistry. 2013 Nov 12;52(45):8126-38. doi: 10.1021/bi400638s. Epub 2013 Oct 28. Biochemistry. 2013. PMID: 24128140 Free PMC article.
References
-
- Caspers G. J., Leunissen J. A., de Jong W. W. (1995) The expanding small heat shock protein family, and structure predictions of the conserved “α-crystallin domain.” J. Mol. Evol. 40, 238–248 - PubMed
-
- de Jong W. W., Caspers G. J., Leunissen J. A. (1998) Genealogy of the α-crystallin-small heat shock protein superfamily. Int. J. Biol. Macromol. 22, 151–162 - PubMed
-
- Jakob U., Gaestel M., Engel K., Buchner J. (1993) Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268, 1517–1520 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

