The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health
- PMID: 22308102
- PMCID: PMC3269821
- DOI: 10.1016/j.hal.2011.10.012
The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health
Abstract
The dinoflagellate genus Alexandrium is one of the major harmful algal bloom (HAB) genera with respect to the diversity, magnitude and consequences of blooms. The ability of Alexandrium to colonize multiple habitats and to persist over large regions through time is testimony to the adaptability and resilience of this group of species. Three different families of toxins, as well as an as yet incompletely characterized suite of allelochemicals are produced among Alexandrium species. Nutritional strategies are equally diverse, including the ability to utilize a range of inorganic and organic nutrient sources, and feeding by ingestion of other organisms. Many Alexandrium species have complex life histories that include sexuality and often, but not always, cyst formation, which is characteristic of a meroplanktonic life strategy and offers considerable ecological advantages. Due to the public health and ecosystem impacts of Alexandrium blooms, the genus has been extensively studied, and there exists a broad knowledge base that ranges from taxonomy and phylogeny through genomics and toxin biosynthesis to bloom dynamics and modeling. Here we present a review of the genus Alexandrium, focusing on the major toxic and otherwise harmful species.
Figures

 ), A. minutum (
), A. minutum (
 ), A. tamutum (
), A. tamutum (
 ), A. peruvianum/A. ostenfeldii (
), A. peruvianum/A. ostenfeldii (
 ), A. insuetum (
), A. insuetum (
 ), A. margalefi (
), A. margalefi (
 ), A. pseudogonyaulax (
), A. pseudogonyaulax (
 ), A. taylori (
), A. taylori (
 ), A. affine (
), A. affine (
 ), A. catenella Group VI (◆), A. tamarense Group II (
), A. catenella Group VI (◆), A. tamarense Group II (
 ), and Group III (
), and Group III (
 ).
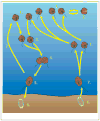
).
References
-
- Abadie E, Amzil Z, Belin C, Comps M-A, Elziere-Papayanni P, Lassus P, Le Bec C, Marcaillou-Le Baut C, Nezan E, Poggi R. Contamination de l’etang de Thau par Alexandrium tamarense: Épisode de novembre à décembre 1998. Plouzané, France: Ifremer; 1999. p. 44.
-
- Achiha H, Iwasaki H. Growth characteristics of the toxic dinoflagellate, Alexandrium tamarense. Jpn J Phycol. 1990;38:31–59.
-
- Adachi M, Kanno T, Matsubara T, Nishijima T, Itakura S, Yamaguchi M. Promotion of cyst formation in the toxic dinoflagellate Alexandrium (Dinophyceae) by natural bacterial assemblages from Hiroshima Bay, Japan. Mar Ecol Prog Ser. 1999;191:175–185.
-
- Adachi M, Sako Y, Ishida Y. Restriction fragment length polymorphism of ribosomal DNA internal transcribed spacer and 5.8S regions in Japanese Alexandrium species (Dinophyceae) J Phycol. 1994;30:857–63.
-
- Alpermann T, Beszteri B, John U, Tillmann U, Cembella A. Implications of life-history transitions on the population genetic structure of the toxigenic marine dinoflagellate Alexandrium tamarense. Mol Ecol. 2009;18:2122–2133. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

