Amazing results with hydroxyurea therapy in chronic hepatitis B: a preliminary report
- PMID: 22308141
- PMCID: PMC3269086
Amazing results with hydroxyurea therapy in chronic hepatitis B: a preliminary report
Abstract
Background and aims: To introduce the possible role of hydroxyurea (HU), a well-characterized antineoplastic drug with established antiviral effects, in the treatment of chronic hepatitis B.
Methods: Four antiretroviral-naïve patients with chronic hepatitis B were enrolled in this limited pilot trial, and given 1000 mg/day of hydroxyurea for 4 weeks; then, the administration of the drug was suspended for 4 weeks. A clinical study and laboratory safety assessments and measurements of viral load were made at baseline, after drug therapy, and after one month suspension of the treatment
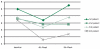
Results: All 4 patients showed a significant decrease in viral load after 4 weeks of hydroxyurea therapy and the viral load of 2 patients increased again after a 4-week suspension of hydroxyurea
Conclusions: Our data demonstrate that the old low-cost antineoplastic drug, hydroxyurea, efficiently blocks hepatitis B virus (HBV) replication. We suggest that HU will play an important role in the treatment of chronic hepatitis in the foreseeable future. Further studies including those that evaluate optimal dosing in long-term use will continue to define the role of HU in the treatment of HBV infection alone or in combination with other antiviral drugs.
Keywords: Antiviral Therapy; Chronic Hepatitis B; HBV; Hydroxyurea.
Figures
Similar articles
-
Treatment of chronic hepatitis B: case selection and duration of therapy.J Gastroenterol Hepatol. 2002 Apr;17(4):409-14. doi: 10.1046/j.1440-1746.2002.02767.x. J Gastroenterol Hepatol. 2002. PMID: 11982721 Review.
-
Week 4 viral load predicts long-term suppression of hepatitis B virus DNA during antiviral therapy: improving hepatitis B treatment in the real world.Intern Med J. 2017 Jan;47(1):50-56. doi: 10.1111/imj.13244. Intern Med J. 2017. PMID: 27571991
-
Combination therapy with lamivudine and famciclovir for chronic hepatitis B infection.Clin Gastroenterol Hepatol. 2004 Apr;2(4):330-6. doi: 10.1016/s1542-3565(04)00063-1. Clin Gastroenterol Hepatol. 2004. PMID: 15067628 Clinical Trial.
-
Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study.J Hepatol. 2018 Jun;68(6):1123-1128. doi: 10.1016/j.jhep.2018.01.037. Epub 2018 Feb 13. J Hepatol. 2018. PMID: 29452204 Clinical Trial.
-
Perioperative antiviral therapy for chronic hepatitis B-related hepatocellular carcinoma.Hepatobiliary Pancreat Dis Int. 2013 Jun;12(3):251-5. doi: 10.1016/s1499-3872(13)60041-7. Hepatobiliary Pancreat Dis Int. 2013. PMID: 23742769 Review.
Cited by
-
Oral disease-modifying antirheumatic drugs and immunosuppressants with antiviral potential, including SARS-CoV-2 infection: a review.Ther Adv Musculoskelet Dis. 2020 Sep 3;12:1759720X20947296. doi: 10.1177/1759720X20947296. eCollection 2020. Ther Adv Musculoskelet Dis. 2020. PMID: 32952617 Free PMC article.
-
Nitric oxide and viral infection: Recent developments in antiviral therapies and platforms.Appl Mater Today. 2021 Mar;22:100887. doi: 10.1016/j.apmt.2020.100887. Epub 2020 Dec 5. Appl Mater Today. 2021. PMID: 38620577 Free PMC article. Review.
-
Hydroxyurea: A useful adjunct to the standard antiviral therapy in chronic hepatitis B.Hepat Mon. 2011 Mar;11(3):210. Hepat Mon. 2011. PMID: 22087148 Free PMC article. No abstract available.
References
-
- Alavian SM, Hajarizadeh B, Ahmadzad-AslM , Kabir A, Bagheri-Lankarani K. Hepatitis B Virus Infection in Iran: A Systematic Review. Hepat Mon. 2008;8(4):281–94.
-
- Donehower RC. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992;19(3 Suppl 9):11–9. - PubMed
-
- Nordenskjold A, Krakoff IH. Effects of hydroxyurea on polyoma virus replication. Cancer Res. 1968;28(9):1686–91. - PubMed
-
- Ravot E, Lisziewicz J, Lori F. New uses for old drugs in HIV infection:the role of hydroxyurea, cyclosporin and thalidomide. Drugs. 1999;58(6):953–63. - PubMed
LinkOut - more resources
Full Text Sources